The next two labs deal with the inhibition, destruction, and removal of microorganisms. Control of microorganisms is essential in order to prevent the transmission of diseases and infection, stop decomposition and spoilage, and prevent unwanted microbial contamination.
Microorganisms are controlled by means of physical agents and chemical agents. Physical agents include such methods of control as high or low temperature, desiccation, osmotic pressure, radiation, and filtration. Control by chemical agents refers to the use of disinfectants, antiseptics, antibiotics, and chemotherapeutic antimicrobial chemicals.
Basic terms used in discussing the control of microorganisms include:
1. Sterilization
Sterilization is the process of destroying all living organisms and viruses. A sterile object is one free of all life forms, including bacterial endospores, as well as viruses.2. Disinfection
Disinfection is the elimination of microorganisms, but not necessarily endospores, from inanimate objects or surfaces.3. Decontamination
Decontamination is the treatment of an object or inanimate surface to make it safe to handle.4. Disinfectant
A disinfectant is an agents used to disinfect inanimate objects but generally to toxic to use on human tissues.5. Antiseptic
An antiseptic is an agent that kills or inhibits growth of microbes but is safe to use on human tissue.6. Sanitizer
A sanitizer is an agent that reduces, but may not eliminate, microbial numbers to a safe level.7. Antibiotic
An antibiotic is a metabolic product produced by one microorganism that inhibits or kills other microorganisms.8. Chemotherapeutic antimicrobial chemical
Chemotherapeutic antimicrobial chemicals are synthetic chemicals that can be used therapeutically.9. Cidal
An agent that is cidal in action will kill microorganisms and viruses.10. Static
An agent that is static in action will inhibit the growth of microorganisms.
These two labs will demonstrate the control of microorganisms with physical agents, disinfectants and antiseptics, and antimicrobial chemotherapeutic agents. Keep in mind that when evaluating or choosing a method of controlling microorganisms, you must consider the following factors which may influence antimicrobial activity:
1. the concentration and kind of a chemical agent used;
2. the intensity and nature of a physical agent used;
3. the length of exposure to the agent;
4. the temperature at which the agent is used;
5. the number of microorganisms present;
6. the organism itself; and
7. the nature of the material bearing the microorganism.
B. TEMPERATURE
Microorganisms have a minimum, an optimum, and a maximum temperature for growth. Temperatures below the minimum usually have a static action on microorganisms. They inhibit microbial growth by slowing down metabolism but do not necessarily kill the organism. Temperatures above the maximum usually have a cidal action, since they denature microbial enzymes and other proteins. Temperature is a very common and effective way of controlling microorganisms.
1. High Temperature
Vegetative microorganisms can generally be killed at temperatures from 50°C to 70°C with moist heat. Bacterial endospores, however, are very resistant to heat and extended exposure to much higher temperature is necessary for their destruction. High temperature may be applied as either moist heat or dry heat. The lowest temperature at which all microorganisms in a liquid suspension will be killed in 10 minutes is referred to as the thermal death point.
a. Moist heat
Moist heat is generally more effective than dry heat for killing microorganisms because of its ability to penetrate microbial cells. Moist heat kills microorganisms by denaturing their proteins (causes proteins and enzymes to lose their three-dimensional functional shape). It also may melt lipids in cytoplasmic membranes.
1. Autoclaving
Autoclaving employs steam under pressure. Water normally boils at 100°C; however, when put under pressure, water boils at a higher temperature. During autoclaving, the materials to be sterilized are placed under 15 pounds per square inch of pressure in a pressure-cooker type of apparatus. When placed under 15 pounds of pressure, the boiling point of water is raised to 121°C, a temperature sufficient to kill bacterial endospores.
The time the material is left in the autoclave varies with the nature and amount of material being sterilized. Given sufficient time (generally 15-45 minutes), autoclaving is cidal for both vegetative organisms and endospores, and is the most common method of sterilization for materials not damaged by heat.
2. Boiling water
Boiling water (100°C) will generally kill vegetative cells after about 10 minutes of exposure. However, certain viruses, such as the hepatitis viruses, may survive exposure to boiling water for up to 30 minutes, and endospores of certain Clostridium and Bacillus species may survive even hours of boiling.
b. Dry heat
Dry heat kills microorganisms through a process of protein oxidation rather than protein coagulation. Examples of dry heat include:
1. Hot air sterilization
Microbiological ovens employ very high dry temperatures: 171°C for 1 hour; 160°C for 2 hours or longer; or 121°C for 16 hours or longer depending on the volume. They are generally used only for sterilizing glassware, metal instruments, and other inert materials like oils and powders that are not damaged by excessive temperature.
2. Incineration
Incinerators are used to destroy disposable or expendable materials by burning. We also sterilize our inoculating loops by incineration.
c. Pasteurization
Pasteurization is the mild heating of milk and other materials to kill particular spoilage organisms or pathogens. It does not, however, kill all organisms. Milk is usually pasteurized by heating to 71°C for at least 15 seconds in the flash method or 63-66°C for 30 minutes in the holding method.
2. Low Temperature
Low temperature inhibits microbial growth by slowing down microbial metabolism. Examples include refrigeration and freezing. Refrigeration at 5°C slows the growth of microorganisms and keeps food fresh for a few days. Freezing at -10°C stops microbial growth, but generally does not kill microorganisms, and keeps food fresh for several months.
C. DESICCATION
Desiccation, or drying, generally has a static effect on microorganisms. Lack of water inhibits the action of microbial enzymes. Dehydrated and freeze-dried foods, for example, do not require refrigeration because the absence of water inhibits microbial growth.
D. OSMOTIC PRESSURE
Microorganisms, in their natural environments, are constantly faced with alterations in osmotic pressure. Water tends to flow through semipermeable membranes, such as the cytoplasmic membrane of microorganisms, towards the side with a higher concentration of dissolved materials (solute). In other words, water moves from greater water (lower solute) concentration to lesser water (greater solute) concentration.
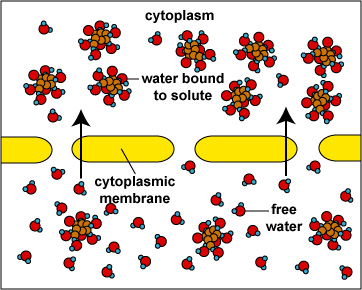
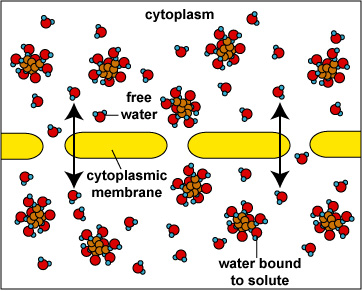
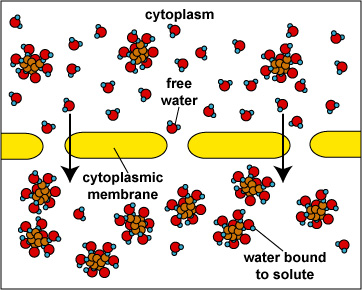
When the concentration of dissolved materials or solute is higher inside the cell than it is outside, the cell is said to be in a hypotonic environment and water will flow into the cell (Fig. 1). The rigid cell walls of bacteria and fungi, however, prevent bursting or plasmoptysis. If the concentration of solute is the same both inside and outside the cell, the cell is said to be in an isotonic environment (Fig. 2). Water flows equally in and out of the cell. Hypotonic and isotonic environments are not usually harmful to microorganisms. However, if the concentration of dissolved materials or solute is higher outside of the cell than inside, then the cell is in a hypertonic environment (Fig. 3). Under this condition, water flows out of the cell, resulting in shrinkage of the cytoplasmic membrane or plasmolysis. Under such conditions, the cell becomes dehydrated and its growth is inhibited.
Fig. 1:
Osmosis (Cell in Hypotonic Environment)Fig. 2:
Osmosis (Cell in an Isotonic Environment)Fig. 3:
Osmosis (Cell in Hypertonic Environment)Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
The canning of jams or preserves with a high sugar concentration inhibits bacterial growth through hypertonicity. The same effect is obtained by salt-curing meats or placing foods in a salt brine. This static action of osmotic pressure thus prevents bacterial decomposition of the food. Molds, on the other hand, are more tolerant of hypertonicity. Foods, such as those mentioned above, tend to become overgrown with molds unless they are first sealed to exclude oxygen. (Molds are aerobic.)
For more information on osmosis and the cytoplasmic membrane, see the following CourseArc lesson:
E. RADIATION
1. Ultraviolet Radiation
The ultraviolet portion of the light spectrum includes all radiations with wavelengths from 100 nm to 400 nm. It has low wave-length and low energy. The microbicidal activity of ultraviolet (UV) light depends on the length of exposure: the longer the exposure the greater the cidal activity. It also depends on the wavelength of UV used. The most cidal wavelengths of UV light lie in the 260 nm - 270 nm range where it is absorbed by nucleic acid.
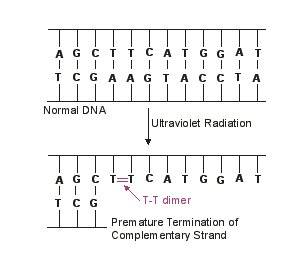
In terms of its mode of action, UV light is absorbed by microbial DNA and causes adjacent thymine bases on the same DNA strand to covalently bond together, forming what are called thymine-thymine dimers (see Fig. 4). As the DNA replicates, nucleotides do not complementary base pair with the thymine dimers and this terminates the replication of that DNA strand. However, most of the damage from UV radiation actually comes from the cell trying to repair the damage to the DNA by a process called SOS repair. In very heavily damaged DNA containing large numbers of thymine dimers, a process called SOS repair is activated as kind of a last ditch effort to repair the DNA. In this process, a gene product of the SOS system binds to DNA polymerase allowing it to synthesize new DNA across the damaged DNA. However, this altered DNA polymerase loses its proofreading ability resulting in the synthesis of DNA that itself now contains many misincorporated bases. In other words, UV radiation causes mutation and can lead to faulty protein synthesis. With sufficient mutation, bacterial metabolism is blocked and the organism dies. Agents such as UV radiation that cause high rates of mutation are called mutagens.
Fig. 4
Mutation as a Result of Exposure to Ultraviolet RadiationGary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
The effect of this inproper base pairing may be reversed to some extent by exposing the bacteria to strong visible light immediately after exposure to the UV light. The visible light activates an enzyme that breaks the bond that joins the thymine bases, thus enabling correct complementary base pairing to again take place. This process is called photoreactivation.
UV lights are frequently used to reduce the microbial populations in hospital operating rooms and sinks, aseptic filling rooms of pharmaceutical companies, in microbiological hoods, and in the processing equipment used by the food and dairy industries.
An important consideration when using UV radiation is that it has very poor penetrating power. Only microorganisms on the surface of a material that are exposed directly to the radiation are susceptible to destruction and bacterial endospores are more resistant to ultraviolet radiation. UV radiation can also damage the eyes, cause burns, and cause mutation in cells of the skin.
2. Ionizing Radiation
Ionizing radiation, such as X-rays and gamma rays, has much more energy and penetrating power than ultraviolet radiation. It ionizes water and other molecules to form radicals (molecular fragments with unpaired electrons) that can disrupt DNA molecules and proteins. It is often used to sterilize pharmaceuticals and disposable medical supplies such as syringes, surgical gloves, catheters, sutures, and petri plates. It can also be used to retard spoilage in seafoods, meats, poultry, and fruits.
For more information on mutation, see the following CourseArc lesson:
F. FILTRATION
Microbiological membrane filters provide a useful way of sterilizing materials such as vaccines, antibiotic solutions, animal sera, enzyme solutions, vitamin solutions, and other solutions that may be damaged or denatured by high temperatures or chemical agents. The filters contain pores small enough to prevent the passage of microbes but large enough to allow the organism-free fluid to pass through. The liquid is then collected in a sterile flask (Fig. 5). Filters with a pore diameter from 25 nm to 0.45 µm are usually used in this procedure. Filters can also be used to remove microorganisms from water and air for microbiological testing (see Appendix E).
Fig. 5
Micropore Filtration ApparatusGary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
PROCEDURE
| Aseptic Technique Tips |
| Aseptic Technique: Inoculation of broth tubes, slant tubes, and stab tubes |
A. OSMOTIC PRESSURE
MEDIA
2 plates of Trypticase Soy agar, 2 plates of 5% glucose agar, 2 plates of 10% glucose agar, 2 plates of 25% glucose agar, 2 plates of 5% NaCl agar, 2 plates of 10% NaCl agar, and 2 plates of 15% NaCl agar.
ORGANISMS
Trypticase Soy broth cultures of Escherichia coli and Staphylococcus aureus; a spore suspension of the mold Aspergillus niger.
A. OSMOTIC PRESSURE PROCEDURE (to be done by tables)
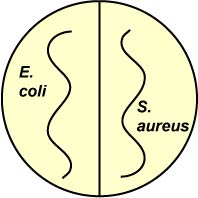
1. Divide one plate of each of the following media in half. Using your inoculating loop, streak one half of each plate with E. coli and the other half with S. aureus (see Fig. 6). Incubate upside down and stacked in the petri plate holder on the shelf of the 37°C incubator corresponding to your lab section until the next lab period.
a. Trypticase Soy agar (control)
b. Trypticase Soy agar with 5% glucose
c. Trypticase Soy agar with 10% glucose
d. Trypticase Soy agar with 25% glucose
e. Trypticase Soy agar with 5% NaCl
f. Trypticase Soy agar with 10% NaCl
g. Trypticase Soy agar with 15% NaCl
Fig. 6: Inoculation of Salt and Sugar Plates with Escherichia coli and Staphylococcus aureus
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
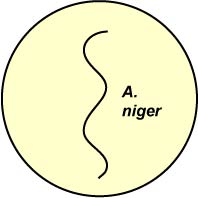
2. Using a sterile swab, streak one plate of each of the following media with a spore suspension of the mold A. niger (see Fig. 7). Incubate the plates upside down at room temperature for 1 week.
a. Trypticase Soy agar (control)
b. Trypticase Soy agar with 5% glucose
c. Trypticase Soy agar with 10% glucose
d. Trypticase Soy agar with 25% glucose
e. Trypticase Soy agar with 5% NaCl
f. Trypticase Soy agar with 10% NaCl
g. Trypticase Soy agar with 15% NaCl
Fig. 7: Inoculation of Salt and Sugar Plates with Aspergillus niger
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
B. ULTRAVIOLET RADIATION
MEDIA
5 plates of Trypticase Soy agar
ORGANISM
Trypticase Soy broth culture of Serratia marcescens
ULTRAVIOLET RADIATION PROCEDURE (to be done by tables)
1. Using sterile swabs, streak all 5 Trypticase Soy agar plates with S. marcescens as follows:
a. Dip the swab into the culture.
b. Remove all of the excess liquid by pressing the swab against the side of the tube.
c. Streak the plate so as to cover the entire agar surface with organisms.
2. Expose 3 of the plates to UV light as follows:
a. Remove the lid of each plate and place a piece of cardboard with the letter "V" cut out of it over the top of the agar.
b. Expose the first plate to UV light for 1 second, the second plate for 3 seconds, and the third plate for 5 seconds.
c. Replace the lids and incubate the plates upside down at room temperature until the next lab period.
3. Leaving the lid on, lay the cardboard with the letter "V" cut out over the fourth plate and expose to UV light for 30 seconds. Incubate the plates upside down at room temperature with the other plates.
4. Use the fifth plate as a non-irradiated control and incubate the plates upside down at room temperature with the other plates.
NOTE: Do not look directly at the UV light as it may harm the eyes.
C. FILTRATION
MEDIUM
2 plates of Trypticase Soy agar
ORGANISM
Trypticase Soy broth cultures of Micrococcus luteus
FILTRATION PROCEDURE (demonstration)
1. Using alcohol-flamed forceps, aseptically place a sterile membrane filter into a sterile filtration device.
2. Pour the culture of M. luteus into the top of the filter set-up.
3. Vacuum until all the liquid passes through the filter into the sterile flask.
4. With alcohol-flamed forceps, remove the filter and place it organism-side-up on the surface of a Trypticase Soy agar plate.
5. Using a sterile swab, streak the surface of another Trypticase Soy agar plate with the filtrate from the flask.
6. Incubate the plates at 37°C until the next lab period.
RESULTS
A. Osmotic Pressure
Observe the 2 sets of plates from the osmotic pressure experiment and record the results below.
| Plate | Escherichia coli | Staphylococcus aureus | Aspergillus niger |
|---|---|---|---|
| Control | +++ |
+++ |
+++ |
| 5% NaCl | |||
| 10% NaCl | |||
| 15% NaCl | |||
| 5% glucose | |||
| 10% glucose | |||
| 25% glucose |
+ = Scant growth
++ = Moderate growth
+++ = Abundant growth
- = No growth
| Escherichia coli
and Staphylococcus aureus |
Aspergillus niger |
|---|---|
Conclusions:
B. Ultraviolet Radiation
1. Make drawings of the 5 plates from the ultraviolet light experiment.
Conclusions:
2. Observe the plates exposed to UV light for any non-pigmented colonies. Aseptically pick-off one of these nonpigmented colonies and streak it in a plate of Trypticase Soy agar. Incubate at room temperature until the next lab period.
3. After incubation, observe the plate you streaked with the nonpigmented colony.
Does the organism still lack chromogenicity?
What would account for this?
C. FILTRATION
Observe the 2 filtration plates and describe the results below.
|
Plate
containing the filter
(Growth or no growth) |
|
|
Plate
streaked with filtrate
(Growth or no growth) |
Conclusions:
PERFORMANCE OBJECTIVES FOR LAB 17
After completing this lab, the student will be able to perform the following objectives:
A. INTRODUCTION TO THE CONTROL OF MICROORGANISM
1. Define the following terms: sterilization, disinfection, decontamination, static, and cidal.
B. TEMPERATURE
1. State whether moist or dry heat is more effective in controlling microorganisms, and indicate why.
2. State specifically how moist heat kills microorganisms.
3. State two methods of applying moist heat.
4. Briefly describe the process of autoclaving (pressure, time, and temperature).
5. State whether or not boiling is an effective means of sterilization, and indicate why.
6. State specifically how dry heat kills microorganisms.
7. State two methods of applying dry heat.
8. Define pasteurization.
9. State whether low temperature has a static or cidal effect on microorganisms, and indicate why.
C. DESICCATION
1. State whether desiccation has a static or a cidal effect on microorganisms, and indicate how it affects the cell.
D. OSMOTIC PRESSURE
1. Describe osmosis in terms of water flow through a semipermeable membrane.
2. Define the following terms: hypotonic, hypertonic, isotonic, plasmoptysis, and plasmolysis.
3. State why a hypotonic environment does not normally harm bacteria.
4. Describe how bacterial growth is inhibited in jams and salt-cured meats.
5. State whether hypertonicity has a static or a cidal effect on microorganisms.
E. RADIATION
1. State how the wavelength and the length of exposure influence the bacteriocidal effect of UV light.
2. Describe specifically how UV light kills microorganisms.
3. State why UV light is only useful as a means of controlling surface contaminants and give several practical applications.
4. Describe how ionizing radiation kills microorganisms and state several common applications.
F. FILTRATION
1. State the concept behind sterilizing solutions with micropore membrane filters.
2. State why filters are preferred over autoclaving for such materials as vaccines, antibiotic solutions, sera, and enzyme solutions.
SELF-QUIZ

Microbiology Laboratory Manual by Gary E. Kaiser, PhD, Professor of Microbiology
is licensed under a Creative Commons Attribution 4.0 International License.
Last updated:August, 2023