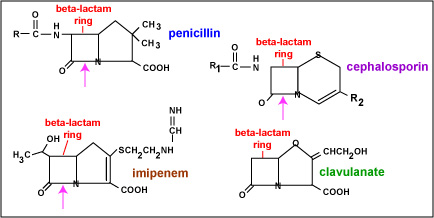
Fig. 5: Beta-Lactam Rings of Penicillins, Cephalosporins, Imipenem, and Clavulanate
Penicillins, monobactams, carbapenems, imipenems, and cephalosporins are known chemically as beta-lactam antibiotics because as part of their chemical structure they contain what is know as a beta-lactam ring. The above illustration shows the basic structure of penicillin, cephalosporin, imipenem, and clavulanate. R represents sites where different chemical side chains attach, depending on the particular antibiotic.
Some bacteria produce various beta-lactamases, enzymes that break the beta-lactam ring at the site indicated by the arrows, and are able to inactivate some forms of these drugs.
Clavulanate and sulbactam are drugs that resemble beta-lactam antibiotics. These agents are sometimes added to beta-lactam antibiotics to tie up and, therefore, inactivate the bacterial beta-lactamases.