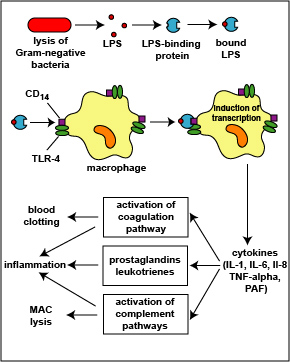
The lysis of Gram-negative bacteria causes them to release lipopolysaccharide (LPS; endotoxin) from the outer membrane of their cell wall. The LPS binds to a LPS-binding protein circulating in the blood and this complex, in turn, binds to a receptor molecule (CD14) found on the surface of body defense cells called macrophages. This is thought to promote the ability of the toll-like receptor TLR-4 to respond to the LPS, triggering the macrophage to release various defense regulatory chemicals called cytokines, including IL-1, IL-6, IL-8, TNF-alpha, and PAF. The cytokines then bind to cytokine receptors on target cells and initiate inflammation and activate both the complement pathways and the coagulation pathway. LPS can also bind directly to TLR-4 molecules.
(LPS, lipopolysaccharide;TLR, toll-like receptor; IL-1, interleukin-1; IL-6, interleukin-6; IL-8, interleukin-8, TNF-alpha, tumor necrosis factor-alpha; PAF, platelet-activating factor.) This will be discussed in greater detail under Bacterial Pathogenicity.
Illustration of Physiologic Action of
Lipopolysaccharide (LPS)
from the Gram-Negative Cell Wall .jpg by Gary E. Kaiser, Ph.D.
Professor of Microbiology,
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 4.0 International License.
Based on a work at https://cwoer.ccbcmd.edu/science/microbiology/index_gos.html.
Last updated: August, 2019
Please send comments and inquiries to Dr.
Gary Kaiser