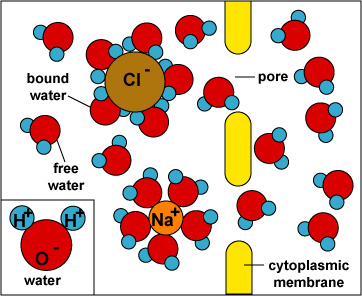

When an ionic solute such as NaCl dissolves in water, the Na+ ion attracts the partial negative charge of the oxygen atom in the water molecule while the Cl- ion attracts the partial positive charge of the warter's hydrogen. While free, unbound water molecules are small enough to pass through membrane pores, water molecules bound to solute are not.
Last updated: August, 2019
Please send comments and inquiries to Dr.
Gary Kaiser