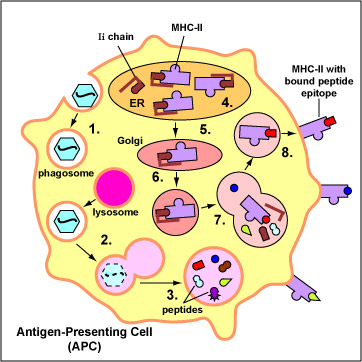
Exogenous antigens are those from outside cells of the body. Examples include bacteria, free viruses, yeasts, protozoa, and toxins. These exogenous antigens enter antigen-presenting cells or APCs (macrophages, dendritic cells, and B-lymphocytes) through phagocytosis. The microbes are engulfed and placed in a phagosome. After lysosomes fuse with the phagosome, protein antigens are degraded by proteases into a series of peptides. These peptides eventually bind to grooves in MHC-II milecules and are transported to the surface of the APC. T4-lymphocytes are then able to recognize peptide/MHC-II complexes by means of their T-cell receptors (TCRs) and CD4 molecules.
1. Exogenous antigens, such as viruses,
are engulfed and placed in a phagosome.
2. Lysosomes fuse with the phagosome forming an phagolysosome.
3. Protein antigens are degraded into a series of peptides.
4. MHC-II molecules are synthesized
in the endoplasmic reticulum and transported to the Golgi complex. Once assembled,
within the endoplasmic reticulum, a protein called the invarient chain (Ii)
attaches to the the peptide-binding groove of the MHC-II molecules and in this
way prevents peptides designated for binding to MHC-I molecules within the ER
from attaching to the MHC-II.
5&6. The MHC-II molecules with bound Ii
chain are now transported to the Golgi complex, and placed in vesicles.
7. The vesicles containing the MHC-II molecules fuse with the peptide-containing
phaglysosomes. The Ii chain
is removed and the peptides are now free to bind to the grooves of the MHC-II
molecules.
8. The MHC-II molecules with bound peptides are transported to the cytoplasmic
membrane where they become anchored. Here, the peptide and MHC-II complexes
can be recognized by T4-lymphocytes by way of TCRs and CD4 molecules having
a complementary shape.
Last updated: August, 2019
Please send comments and inquiries to Dr.
Gary Kaiser