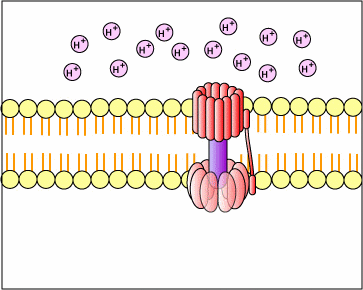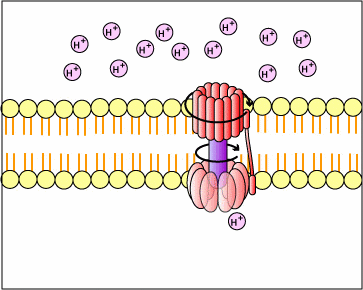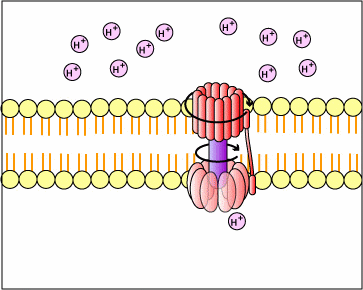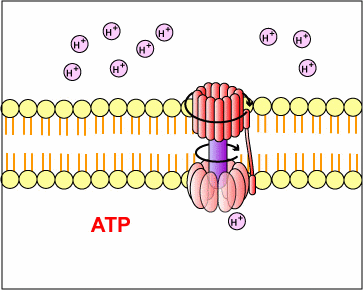
The chemiosmotic theory explains the functioning of electron transport chains. According to this theory, the tranfer of electrons down an electron transport system through a series of oxidation-reduction reactions releases energy . This energy allows certain carriers in the chain to transport hydrogen ions (H+ or protons) across a membrane. As the hydrogen ions accumulate on one side of a membrane, the concentration of hydrogen ions creates an electrochemical gradient or potential difference (voltage) across the membrane. (The fluid on the side of the membrane where the protons accumulate acquires a positive charge; the fluid on the opposite side of the membrane is left with a negative charge.) The energized state of the membrane as a result of this charge separation is called proton motive force or PMF.
This proton motive force provides the energy necessary for enzymes called ATP synthases, also located in the membranes mentioned above, to catalyze the synthesis of ATP from ADP and phosphate. This generation of ATP occurs as the protons cross the membrane through the ATP synthase complexes and re-enter either the bacterial cytoplasm or the matrix of the mitochondria. As the protons move down the concentration gradient through the ATP synthase, the energy released causes the rotor and rod of the ATP synthase to rotate. The mechanical energy from this rotation is converted into chemical energy as phosphate is added to ADP tform ATP.