A. INTRODUCTION TO SEROLOGIC TESTING
The adaptive immune responses refer to the ability of the body (self) to recognize specific foreign antigens (non-self) that threaten its biological integrity. There are two major branches of the adaptive immune responses:
1. Humoral immunity: humoral immunity involves the production of antibody molecules in response to an antigen and is mediated by B-lymphocytes.
2. Cell-mediated immunity: Cell-mediated immunity involves the production of cytotoxic T-lymphocytes, activated macrophages, activated NK cells, and cytokines in response to an antigen and is mediated by T-lymphocytes.
To understand the immune responses we must first understand what is meant by the term antigen. Technically, an antigen is defined as a substance that reacts with antibody molecules and antigen receptors on lymphocytes. An immunogen is an antigen that is recognized by the body as nonself and stimulates an adaptive immune response. For simplicity, both antigens and immunogens are usually referred to as antigens.
Chemically, antigens are large molecular weight proteins (including conjugated proteins such as glycoproteins, lipoproteins, and nucleoproteins) and polysaccharides (including lipopolysaccharides). These protein and polysaccharide antigens are found on the surfaces of viruses and cells, including microbial cells (bacteria, fungi, protozoans) and human cells.
As mentioned above, the B-lymphocytes and T-lymphocytes are the cells that carry out adaptive immune responses. The body recognizes an antigen as foreign when that antigen binds to the surfaces of B-lymphocytes and T-lymphocytes by way of antigen-specific receptors having a shape that corresponds to that of the antigen, similar to interlocking pieces of a puzzle. The antigen receptors on the surfaces of B-lymphocytes are antibody molecules called B-cell receptors or sIg; the receptors on the surfaces of T-lymphocytes are called T-cell receptors (TCRs).
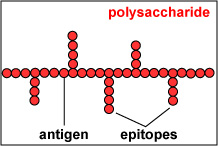
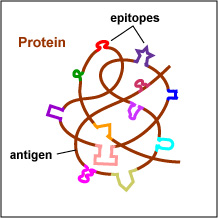
The actual portions or fragments of an antigen that react with receptors on B-lymphocytes and T-lymphocytes, as well as with free antibody molecules, are called epitopes. The size of an epitope is generally thought to be equivalent to 5-15 amino acids or 3-4 sugar residues. Some antigens, such as polysaccharides, usually have many epitopes, but all of the same specificity. This is because polysaccharides may be composed of hundreds of sugars with branching sugar side chains, but usually contain only one or two different sugars. As a result, most "shapes" along the polysaccharide are the same (see Fig. 1). Other antigens such as proteins usually have many epitopes of different specificities. This is because proteins are usually hundreds of amino acids long and are composed of 20 different amino acids. Certain amino acids are able to interact with other amino acids in the protein chain and this causes the protein to fold over upon itself and assume a complex three-dimensional shape. As a result, there are many different "shapes" on the protein (see Fig. 2). That is why proteins are more immunogenic than polysaccharides; they are chemically more complex.
A microbe, such as a single bacterium, has many different proteins (and polysaccharides) on its surface that collectively form its various structures, and each different protein may have many different epitopes. Therefore, immune responses are directed against many different parts or epitopes of the same microbe.
Fig. 1: Epitopes of an Antigen (Polysaccharide)
Fig. 2: Epitopes of an Antigen (Protein)
Polysaccharides have many epitopes but of similar specificities. Proteins have many epitopes of different specificities. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
In terms of infectious diseases, the following may act as antigens:
1.Microbial structures (cell walls, capsules, flagella, pili, viral capsids, envelope-associated glycoproteins, etc.); and
2. Microbial toxins
Certain non-infectious materials may also act as antigens if they are recognized as "non-self" by the body. These include:
1. Allergens (dust, pollen, hair, foods, dander, bee venom, drugs, and other agents causing allergic reactions);
2. Foreign tissues and cells (from transplants and transfusions); and
3. The body's own cells that the body fails to recognize as "normal self" (cancer cells, infected cells, cells involved in autoimmune diseases).
Antibodies or immunoglobulins are specific protein configurations produced by B-lymphocytes and plasma cells in response to a specific antigen and capable of reacting with that antigen. Antibodies are produced in the lymphoid tissue and once produced, are found mainly in the plasma portion of the blood (the liquid fraction of the blood before clotting). Serum is the liquid fraction of the blood after clotting.
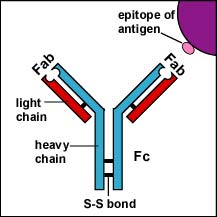
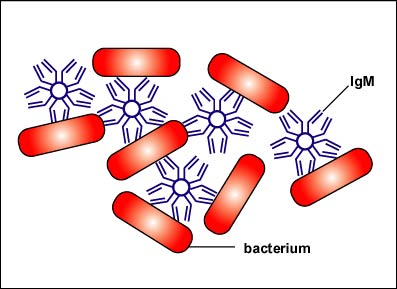
There are 5 classes of human antibodies: IgG, IgM, IgA, IgD, and IgE. The simplest antibodies, such as IgG, IgD, and IgE, are "Y"-shaped macromolecules called monomers composed of four glycoprotein chains. There are two identical heavy chains having a high molecular weight that varies with the class of antibody. In addition, there are two identical light chains of one of two varieties: kappa or gamma. The light chains have a lower molecular weight. The four glycoprotein chains are connected to one another by disulfide (S-S) bonds and noncovalent bonds (see Fig. 3A). Additional S-S bonds fold the individual glycoprotein chains into a number of distinct globular domains. The area where the top of the "Y" joins the bottom is called the hinge. This area is flexible to enable the antibody to bind to pairs of epitopes various distances apart on an antigen.
The two tips of the "Y" monomer are referred to as the Fab portions of the antibody (see Fig. 3A). The first 110 amino acids or first domain of both the heavy and light chain of the Fab region of the antibody provide specificity for binding an epitope on an antigen. The Fab portions provide specificity for binding an epitope on an antigen. The bottom part of the "Y" is called the Fc portion and this part is responsible for the biological activity of the antibody (see Fig. 3A). Depending on the class of antibody, biological activities of the Fc portion of antibodies include the ability to activate the complement pathway (IgG & IgM), bind to phagocytes (IgG, IgA), or bind to mast cells and basophils (IgE).
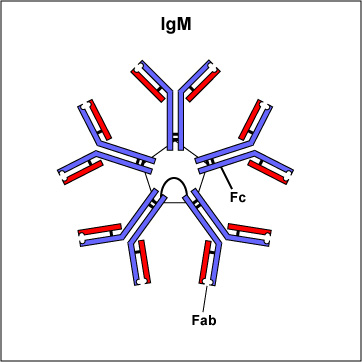
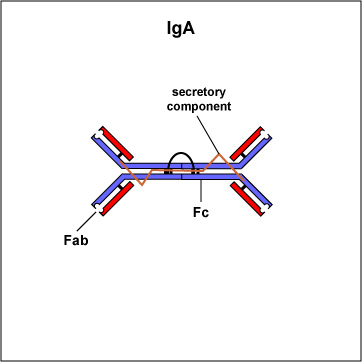
Two classes of antibodies are more complex. IgM is a pentamer (see Fig. 3B), consisting of 5 "Y"-like molecules connected at their Fc portions, and secretory IgA is a dimer consisting of 2 "Y"-like molecules (see Fig. 3C).
Fig. 3A: IgG
Fig. 3B: IgM
Fig. 3C: Secretory IgA
The Fab portion of the antibody has specificity for binding an epitope of an antigen. The Fc portion directs the biological activity of the antibody. IgM is a pentamer and, therefore, has 10 Fab sites. Secretory IgA is a dimer and has 4 Fab sites. A secretory component helps protect it from digestion in body secretions. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
For more information on antigens, antibodies, and antibody production, see the following CourseArc lessons:
Serology refers to using antigen-antibody reactions in the laboratory for diagnostic purposes. Its name comes from the fact that serum, the liquid portion of the blood where antibodies are found is used in testing. Serologic testing may be used in the clinical laboratory in two distinct ways:
a. To identify unknown antigens (such as microorganisms). This is called direct serologic testing. Direct serologic testing uses a preparation known antibodies, called antiserum, to identify an unknown antigen such as a microorganism.
b. To detect antibodies being made against a specific antigen in the patient's serum. This is called indirect serologic testing. Indirect serologic testing is the procedure by which antibodies in a person's serum being made by that individual against an antigen associated with a particular disease are detected using a known antigen.
B. DIRECT SEROLOGIC TESTING: USING ANTIGEN-ANTIBODY REACTIONS IN THE LABORATORY TO IDENTIFY UNKNOWN ANTIGENS SUCH AS MICROORGANISMS.
This type of serologic testing employs known antiserum (serum containing specific known antibodies). The preparation of known antibodies is prepared in one of two ways: in animals or by hybridoma cells.
1. Preparation of known antisera in animals.
Preparation of known antiserum in animals involves inoculating animals with specific known antigens such as a specific strain of a bacterium. After the animal's immune responses have had time to produce antibodies against that antigen, the animal is bled and the blood is allowed to clot. The resulting liquid portion of the blood is the serum and it will contain antibodies specific for the injected antigen.
However, one of the problems of using antibodies prepared in animals (by injecting the animal with a specific antigen and collecting the serum after antibodies are produced) is that up to 90% of the antibodies in the animal's serum may be antibodies the animal has made "on its own" against environmental antigens, rather than those made against the injected antigen. The development of monoclonal antibody technique has largely solved that problem.
2. Preparation of known antibodies by monoclonal antibody technique.
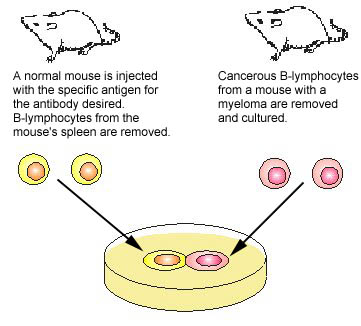
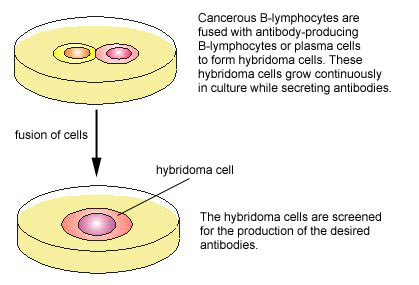
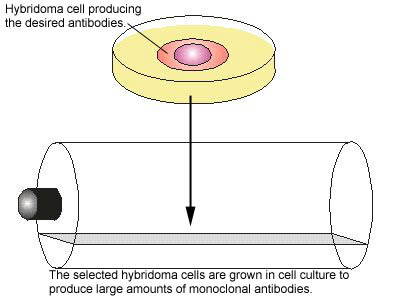
One of the major breakthroughs in immunology occurred when monoclonal antibody technique was developed. Monoclonal antibodies are antibodies of a single specific type. In this technique, an animal is injected with the specific antigen (see Fig. 4, step 1) for the antibody desired. After appropriate time for antibody production, the animal's spleen is removed. The spleen is rich in plasma cells and each plasma cell produces only one specific type of antibody. However, plasma cells will not grow artificially in cell culture. Therefore, a plasma cell producing the desired antibody is fused with a myeloma cell ,a cancer cell from bone marrow which will grow rapidly in cell culture, to produce a hybridoma cell (see Fig. 4, step 2). The hybridoma cell has the characteristics of both parent cells. It will produce the specific antibodies like the plasma cell and will also grow readily in cell culture like the myeloma cell. The hybridoma cells are grown artificially in huge vats where they produce large quantities of the specific antibody (see Fig. 4, step 3).
Fig. 4: Production of Monoclonal Antibodies, Step-1
Fig. 4: Production of Monoclonal Antibodies, Step-2
Fig. 4: Production of Monoclonal Antibodies, Step-3
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Monoclonal antibodies are now used routinely in medical research and diagnostic serology and are being used experimentally in treating certain cancers and a few other diseases.
3. The concept and general procedure for direct serologic testing.
The concept and general procedure for using antigen-antibody reactions to identify unknown antigens are as follows:
- Concept:
This testing is based on the fact that antigen- antibody reactions are very specific. Antibodies usually react only with the antigen that stimulated their production in the first place, and are just as specific as an enzyme-substrate reaction. Because of this, one can use known antiserum (prepared by animal inoculation or monoclonal antibody technique as discussed above) to identify unknown antigens such as a microorganism.
- General Procedure:
A suspension of the unknown antigen to be identified is mixed with known antiserum for that antigen. One then looks for an antigen-antibody reaction.
Examples of serologic tests used to identify unknown microorganisms include the serological typing of Shigella and Salmonella, the Lancefield typing of beta streptococci, and the serological identification of Neisseria gonorrhoeae and Neisseria meningitidis. Serological tests used to identify antigens which are not microorganisms include blood typing, tissue typing, and pregnancy testing.
4. Examples of direct serologic testing to identify unknown antigens
As stated above, this type of serologic testing uses known antiserum (antibodies) to identify unknown antigens. Four such tests will be looked at in lab today.
a. Serological Typing of Shigella
There are four different serological subgroups of Shigella, each corresponding to a different species:
- subgroup A = Shigella dysenteriae
- subgroup B = Shigella flexneri
- subgroup C = Shigella boydii
- subgroup D = Shigella sonnei
Known antiserum is available for each of the 4 subgroups of Shigella listed above and contains antibodies against the cell wall ("O" antigens) of Shigella. The suspected Shigella (the unknown antigen) is placed in each of 4 circles on a slide and a different known antiserum (A, B, C or D) is then added to each circle. A positive antigen-antibody reaction appears as a clumping or agglutination of the Shigella (see Fig. 5).
Fig. 5: Agglutination of Microorganisms
The Fab portions of the antibodies link microorganism together causing agglutination on the slide. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
b. Serological Typing of Streptococci
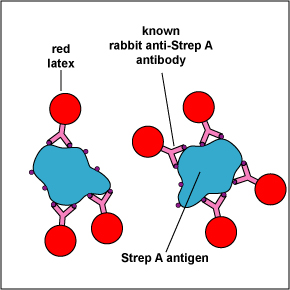
The Clearview® Strep A Exact II Dipstick is an example of a lateral flow immunologic assay. It is is a qualitative serologic test for detecting Group A Streptococcal antigen (the unknown antigen) directly from throat swabs and is used as an aid in diagnosing streptococcal pharyngitis caused by Streptococcus pyogenes (Group A Beta Streptococci).
The test consists of a membrane strip that is precoated with rabbit anti-Strep A monoclonal antibody-red latex conjugate (known antibodies made in rabbits against strep A antigen with red latex particles attached) located in a pad at the beginning of the strip. It is also precoated with rabbit anti-Strep A monoclonal antibody (known antibodies made in rabbits against the strep A antigen but without attached red latex particles) that is immobilized at the test line where the test results are read (see Fig. 6A). The red latex particles attached to the rabbit anti-Strep A antibodies are what ultimately causes the “positive” red band.
Fig. 6A: Serologic Testing to Identify Group A Streptococcus Antigen: The Test Strip
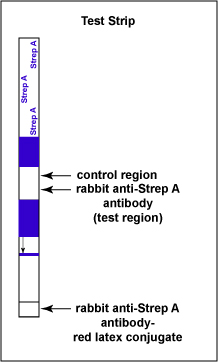
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
The throat swab is placed in an extraction solution that lyses the Streptococcus pyogenes, if present, and exposes the strep A antigen in the bacterial cell wall. When the test strip is immersed in the extracted sample, the Group A Streptococcal antigen extracted from the Streptococcus pyogenes on the throat swab of a person with strep throat begins to move chromatographically up the membrane and binds to the known antibody-red latex conjugate in the pad located at the beginning of the strip, forming a Strep A antigen-antibody complex (see Fig. 6B). This Strep A antigen-antibody complex continues to moves up the membrane to the test line region where the immobilized rabbit anti-Strep A antibodies are located.
Fig. 6B: Serologic Testing to Identify Group A Streptococcus Antigen: Known Antibodies Against Strep A Antigen binding to Strep A Antigens
Cell wall fragments containing Strep A antigen binds to known anti-Strep A antibodies bound to red latex particles. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
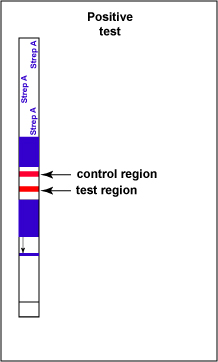
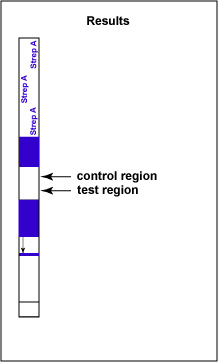
If Group A Streptococcal antigen is present in the throat swab, a red-colored sandwich of known antibody/Strep A antigen/red latex conjugate forms in the test line region of the strip (see Fig. 6C). The control region of the strip has immobilized anti-rabbit antibodies, that is, antibodies made in a different animal against rabbit antibodies. The red color at the control line region appears when molecules of the rabbit anti-Strep A antibody-red latex conjugate not trapped at the test line reach the control area and are stopped by binding to the anti-rabbit antibodies. This indicates that the test is finished. As a result, a positive test for Group A Strep antigen appears as a red band in the test result area and a red band in the control area (see Fig. 6C).
Fig. 6C: Serologic Testing to Identify Group A Streptococcus Antigen: A Positive Test
Note two red stripes. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
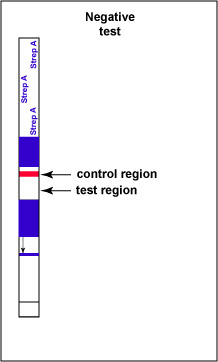
If there is no Group A Streptococcal antigen present in the throat swab no red band appears in the test result region of the strip and a single red band appears in the control line region, indicating a negative test for Group A Strep antigen (see Fig. 6D).
Fig. 6D: Serologic Testing to Identify Group A Streptococcus Antigen: A Negative Test
Note only one red stripe in the test control area. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
c. Serological Testing to Diagnose Pregnancy
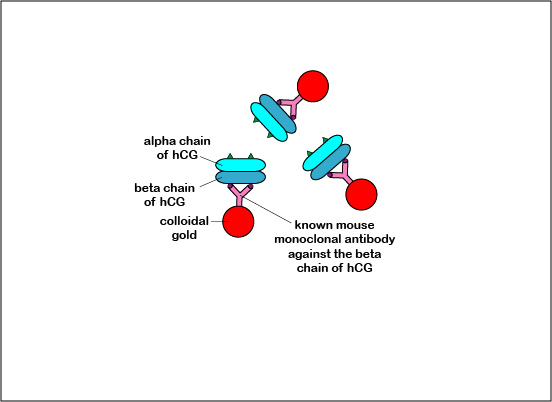
The Alere® hCG Dipstick is an example of a lateral flow immunologic assay. It is a qualitative serologic test for detecting early pregnancy. The hormone human chorionic gonadotropin (hCG), produced by the placenta, appears in the serum and urine of pregnant females. The hCG is composed of two subunits - alpha and beta. The Alere® hCG Dipstick is a one step pregnancy test that detects levels of hCG as low as 25 mlU/ml. Human chorionic gonadotropin (hCG), the unknown antigen for which one is testing, is identified in the urine by using known mouse monoclonal antibodies against the beta subunit of hCG bound to colloidal gold, which is red in color. It also uses known goat polyclonal antibodies against the alpha subunit of hCG which is immobilized on the test result region of the dipstick.
Like the Strep A test mentioned above, this test uses a color immunochromatographic assay to detect the antigen-antibody reaction.The test consists of a membrane strip that is precoated with known mouse anti- beta hCG antibody-colloidal gold conjugate (known antibodies made in mice against the beta chain og hCG with red colloidal gold particles attached) located in a pad at the beginning of the strip. It is also precoated with known goat anti-alpha hCG antibody (known antibodies made in goats against the alpha chain of hCG without attached red colloidal gold) that is immobilized at the test line where the test results are read (see Fig. 7B1). The red colloidal gold particles attached to the mouse anti-alpha hCG antibody is what ultimately causes the “positive” red band in the test area.
Fig. 7B1: Serologic Testing to Identify hCG Antigen: The hCG Test Dipstick
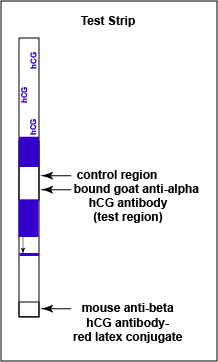
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
When the test strip is immersed in the urine sample, the hCG begins to move chromatographically up the membrane and binds to the red-colored known anti-beta hCG antibody-gold conjugate in the pad located at the beginning of the strip, forming a hCG antigen-antibody complex (see Fig. 7B2). This hCG antigen-antibody complex continues to moves up the membrane to the test line region where the immobilized known goat anti-beta hCG antibodies are located.
Fig. 7B2: Serologic Testing to Identify hCG Antigen
Known monoclonal antibodies against the beta chain of human hCG (bound to colloidal gold) reacting with the beta chain of human hCG. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
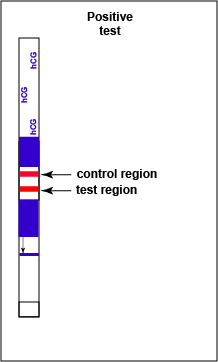
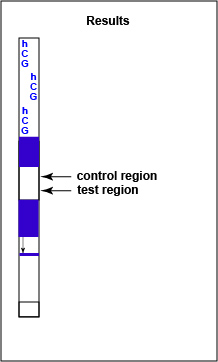
If hCG is present in the urine, a red-colored sandwich of anti-beta antibody/hCG antigen/red gold conjugate anti-alpha antibody forms in the test line region of the strip (see Fig. 7B3). The control region of the strip has immobilized anti-mouse antibodies, that is, antibodies made in a different animal against mouse antibodies. The red color at the control line region appears when molecules of the rabbit anti-hCG antibody-red latex conjugate not trapped at the test line reach the control area and are stopped by binding to the anti-mouse antibodies. The red color at the control line region indicates that the test is finished. As a result, a positive test for hCG antigen appears as a red band in the test result area and a red band in the control area (see Fig. 7B3).
Fig. 7B3: Serologic Testing to Identify hCG Antigen: A Positive Test
Note red band in both the control and the test regions. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
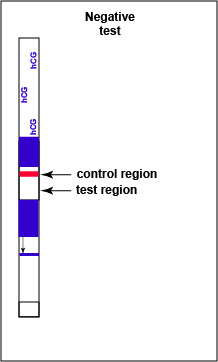
If there is no detectable hCG antigen present in the urine no red band appears in the test result region of the strip and a single red band appears in the control line region, indicating a negative test for hCG antigen (see Fig. 7B4).
Fig. 7B4: Serologic Testing to Identify hCG Antigen: A Negative Test
Note red band in the control region but not in the test region. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
d. COVID-19 Rapid Antigen Tests
Discussion
The various COVID-19 rapid antigen tests are also examples of lateral flow immunologic assays. They are qualitative serologic tests for detecting COVID-19 infection. They detect the nucleocapsid protein antigen (N protein) of SARS-Cov-2, the virus that causes COVID-19. Swabs from the nose or throat are placed in a buffer solution that maintains a pH of 7.4, the pH of the blood. The buffer solutions also contain agents that lyse the viruses, releasing the various viral antigens, including the N protein antigen. The N protein, the unknown antigen for which one is testing, is identified in the sample by using known monoclonal antibodies, made in animals such as rabbits, mice, or chickens, against the N protein and bound to colloidal gold, which is red in color.
Like the Strep A and pregnancy tests mentioned above, this test uses a color immunochromatographic assay to detect the antigen-antibody reaction. The test consists of a membrane strip that is precoated with known anti-N protein antibody-colloidal gold conjugate (known antibodies made in rabbits, mice, chickens, etc. against the N protein of SARS-Cov-2 with red colloidal gold particles attached) located in a pad at the beginning of the strip. In this example discussed here, the anti-N protein antibodies are made using monoclonal antibody technique in rabbits. It is also precoated with known anti-N protein antibody (known antibodies made in rabbits against N protein without attached red colloidal gold) that are immobilized at the test line where the test results are read. The red colloidal gold particles attached to the anti-N protein antibodies is what ultimately causes the positive red band in the test area.
When the patient's sample is added to the sample well, the N-protein antigen, if present, begins to move chromatographically up the membrane and binds to the red-colored known anti-N protein rabbit antibody-gold conjugate in the pad located at the beginning of the test assembly, forming a N protein antigen-antibody-gold conjugate complex. This N protein antigen-antibody complex continues to move up the membrane to the test line region where the immobilized known rabbit anti-N protein antibodies are located.
If N protein is present in the patient's sample, a red-colored sandwich of N protein antigen/anti-N protein rabbit antibody-gold conjugate forms in the test line region of the strip resulting in formation of a red band.
The control region of the strip is precoated with immobilized anti-rabbit antibody antibodies (antibodies made in a different animal against rabbit antibodies). The red color at the control line region appears when molecules of the anti-N protein antibody-red latex conjugate that were not trapped at the test line reach the control area and are stopped by binding to the anti-rabbit antibody antibodies. The red color indicates that the test is finished. As a result, a positive test for COVID-19 antigen appears as a red band in the test result area and a red band in the control area.
If there is no detectable SARS-Cov-2 N protein antigen present in the patient's sample, no red band appears in the test result region of the strip and a single red band appears in the control line region only, indicating a negative antigen test for COVID-19.
e. Identification of Microorganisms Using the Direct Fluorescent Antibody Technique
Certain fluorescent dyes can be chemically attached to the known antibody molecules in antiserum. The known fluorescent antibody is then mixed with the unknown antigen,such as a microorganism, fixed to a slide. After washing, to remove any fluorescent antibody not bound to the antigen, the slide is viewed with a fluorescent microscope.
If the fluorescent antibody reacted with the unknown antigen, the antigen will glow or fluoresce under the fluorescent microscope. If the antibody did not react with the antigen, the antibodies will be washed off the slide and the antigen will not fluoresce.
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
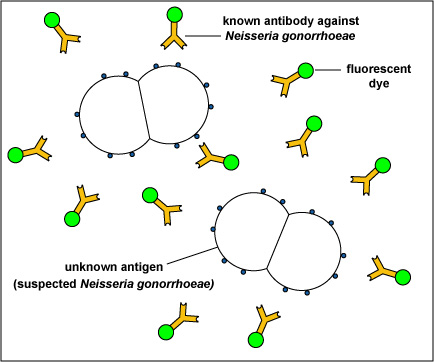
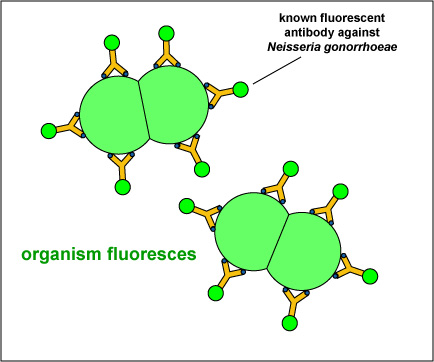
For example, in the direct fluorescent antibody test for Neisseria gonorrhoeae, the unknown antigen, suspected Neisseria gonorrhoeae,is fixed to a microscope slide. Known fluorescent antibodies made against N. gonorrhoeae are then added (see Fig. 8, step 1) and the slide is then washed to remove any fluorescent antibody not bound to the antigen. The slide is then viewed under a fluorescent microscope.
Fig. 8: Direct Fluorescent Antibody Test for Neisseria gonorrhoeae, Step-1
The unknown antigen, suspected Neisseria gonorrhoeae, is fixed to a microscope slide. Known fluorescent antibodies made against N. gonorrhoeae are added. The slide is then washed and viewed under a fluorescent microscope. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
If the unknown antigen is Neisseria gonorrhoeae, the known antibodies against N. gonorrhoeae with attached fluorescent dye will bind to the bacterium and will not wash off. The bacteria will fluoresce when viewed under a fluorescent microscope (see Fig. 8, step 2 and Fig. 9). If the unknown antigen is not N. gonorrhoeae, the known fluorescent antibodies against will wash off the slide and the bacteria will not fluoresce when viewed under a fluorescent microscope.
Fig. 8: Direct Fluorescent Antibody Test for Neisseria gonorrhoeae, Step-2
Fig. 9: A Positive Direct Fluorescent Antibody Test for Neisseria gonorrhoeae
If the unknown antigen is Neisseria gonorrhoeae, the known antibodies against N. gonorrhoeae with attached fluorescent dye will bind to the bacterium and will not wash off. The bacteria will fluoresce when viewed under a fluorescent microscope. 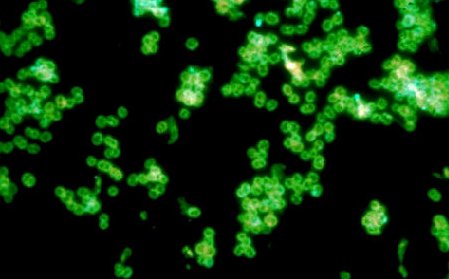
Note green-fluorescent diplococci. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
By Content Providers(s): CDC/Public domain. Courtesy of the Centers for Disease Control and Prevention.
Many bacteria, viruses, and fungi can be identified using this technique.
e. Identification of SAR-CoV-2 RNA Using RT-PCRThe RT-PCR (reverse transcriptase polymerase chain reaction) method is used to diagnose COVID-19 infection by detecting SARS-CoV-2 RNA in a person's nose or throat. The viruses on the swab are lysed and their RNA genomes are purified. Reverse transcriptase is then used to make DNA copies of the RNA viral genome. Polymerase chain reactions are then used to rapidly amplify the number of DNA copies. During this hybridation, probes labeled with a fluorophore bind to their complementary DNA strands.(A fluorophore is a fluorescent chemical compound that can re-emit light upon light excitation.) A TaqMan probe then cleaves the fluorophore bound to the labeled probe and the degree of floresence can then be used to indicate the presence of certain SARS-CoV-2 RNA in the original patient sample.
Medscape articles on infections associated with organisms mentioned in this lab exercise. Registration to access this website is free.
C. INDIRECT SEROLOGIC TESTING: USING ANTIGEN-ANTIBODY REACTIONS IN THE LABORATORY TO INDIRECTLY DIAGNOSE DISEASE BY DETECTING ANTIBODIES IN A PERSON'S SERUM PRODUCED AGAINST A DISEASE ANTIGEN
Indirect serologic testing is the procedure whereby antibodies in a person's serum being made by that individual against an antigen associated with a particular disease are detected using a known antigen.
1. The concept and general procedure for indirect serologic testing.
The concept and general procedure for this type of serological testing are as follows:
- Concept:
This type of testing is based on the fact that antibodies are only produced in response to a specific antigen. In other words, a person will not be producing antibodies against a disease antigen unless that antigen is in the body stimulating antibody production.
- General Procedure:
A sample of the patient's serum (the liquid portion of the blood after clotting and containing antibodies against the disease antigen if the person has or has had the disease) is mixed with the known antigen for that suspected disease. One then looks for an antigen-antibody reaction.
Examples of serologic tests to diagnose disease by the detection of antibodies in the patient's serum include the various serological tests for syphilis or STS (such as the RPR, the VDRL, and the FTA-ABS tests), the tests for infectious mononucleosis, the tests for the Human Immunodeficiency Virus (HIV), the tests for systemic lupus erythematosus, and tests for variety of other viral infections.
2. Qualitative and quantitative serologic tests.
Indirect serologic tests may be qualitative or quantitative. A qualitative test only detects the presence or absence of specific antibodies in the patient's serum and is often used for screening purposes. A quantitative test gives the titer or amount of that antibody in the serum. Titer indicates how far you can dilute the patient's serum and still have it contain enough antibodies to give a detectable antigen-antibody reaction. In other words, the more antibodies being produced by the body, the more you can dilute the person's serum and still see a reaction. Quantitative serological tests are often used to follow the progress of a disease by looking for a rise and subsequent drop in antibody titer.
- A definite clumping of the charcoal particles is reported as reactive (R).
- No clumping is reported as non-reactive (N).
- Agglutination of bacteria is positive.
- No agglutination of bacteria is negative.
3. Examples of indirect serologic tests to detect antibodies in the patient's serum
a. The RPR Test for Syphilis
Syphilis is a sexually transmitted disease caused by the spirochete Treponema pallidum. The RPR (Rapid Plasma Reagin) Card® test is a presumptive serologic screening test for syphilis. The serum of a person with syphilis contains a non-specific anti-lipid antibody (traditionally termed reagin), which is not found in normal serum. The exact nature of the anti-lipid (reagin) antibody is not known but it is thought that a syphilis infection instigates the breakdown of the patient's own tissue cells. Fatty substances which are released then combine with protein from Treponema pallidum to form an antigen which stimulates the body to produce antibodies against both the body's tissue lipids (non-specific or non-treponemal) as well as the T. pallidum protein (specific or treponemal). The RPR Card® test detects the nonspecific antilipid antibody and is referred to as a non-treponemal test for syphilis.
It must be remembered that tests for the presence of these nonspecific antilipid antibodies are meant as a presumptive screening test for syphilis. Similar reagin-like antibodies may also be present as a result of other diseases such as malaria, leprosy, infectious mononucleosis, systemic lupus erythematosus, viral pneumonia, measles, and collagen diseases and may give biologic false-positive results (BFP). Confirming tests should be made for the presence of specific antibodies against the T. pallidum itself. The confirming test for syphilis is the FTA-ABS test discussed below. Any serologic test for syphilis is referred to commonly as an STS (Serological Test for Syphilis).
The known RPR antigen consists of cardiolipin, lecithin, and cholesterol bound to charcoal particles in order to make the reaction visible to the naked eye. If the patient has syphilis, the antilipid antibodies in his or her serum will cross-react with the known RPR lipid antigens giving a visible clumping of the charcoal particles (see Fig. 10).
Fig. 10: Positive RPR Serologic Test for Syphilis
Clumping of the carbon particles indicates the person's serum contains nonspecific antilipid (reagin) antibodies. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
We will do a quantitative RPR Card® test today in lab. Keep in mind that a quantitative test allows one to determine the titer or amount of a certain antibody in the serum. In this test, a constant amount of RPR antigen is added to dilutions of the patient's serum. The most dilute sample of the patient's serum still containing enough antibodies to give a visible antigen-antibody reaction is reported as the titer.
b. Serologic Tests for Infectious Mononucleosis
During infectious mononucleosis, caused by the Epstein-Barr virus (EBV), the body produces non-specific heterophile antibodies which are not found in normal serum. As it turns out, these heterophile antibodies will cross react with glycoprotein antigens found on the surface of red blood cells (RBCs) of various animals, including horses, sheep, and cows, causing the RBCs to agglutinate. These cross-reacting glycoprotein antigens are often called Paul-Bunnell antigens after their discoverers.
The infectious mononucleosis serologic test demonstrated today is a rapid qualitative test for infectious mononucleosis called the ASI Color Mono II Test®. The mono reagent in this test is a suspension of dyed, color-enhanced, preserved horse erythrocytes. The Paul-Bunnel antigens in the cell membrane of these horse erythrocytes are highly specific for mononucleosis heterophile antibodies and act as the "known antigen." Agglutination of the erythrocytes after adding the patient's serum indicates a positive test (see Fig. 11). Quantitative tests may be done to determine the titer of heterophile antibodies and follow the progress of the disease.
Fig. 11: Positive Qualitative Serologic Test for Infectious Mononucleosis
Clumping of the horse erythrocytes containing Paul-Bunnel antigens in their cell membrane indicates the person's serum contains heterophile antibodies. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
c. Serologic Tests for Systemic Lupus Erythematosus (SLE)
Systemic lupus erythematosus or SLE is a systemic autoimmune disease. Immune complexes become deposited between the dermis and the epidermis, and in joints, blood vessels, glomeruli of the kidneys, and the central nervous system. It is four times more common in women than in men. In SLE, autoantibodies are made against components of DNA. This test is specific for the serum anti-deoxyribonucleoprotein antibodies associated with SLE. The known antigen is deoxyribonucleoprotein adsorbed to latex particles to make the reaction more visible to the eye (see Fig. 12). This is a qualitative test used to screen for the presence of the disease and to monitor its course.
Fig. 12: Positive Qualitative Serologic Test for SLE
Clumping of the latex particles indicates the person's serum contains anti-deoxyribonucleoprotein antibodies. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
d. Detecting Antibody Using the Indirect Fluorescent Antibody Technique: The FTA-ABS test for syphilis
The indirect fluorescent antibody technique involves three different reagents:
a. The patient's serum (containing antibodies against the disease antigen if the disease is present)
b. Known antigen for the suspected disease
c. Fluorescent anti-human gamma globulin antibodies (antibodies made in another animal against the Fc portion of human antibodies (see Fig. 13) by injecting an animal with human serum. A fluorescent dye is then chemically attached to the anti-human gamma globulin (anti-HGG) antibodies.
Fig. 13: An Antibody Molecule
The Fab portion of the antibody has specificity for binding an epitope of an antigen. The Fc portion directs the biological activity of the antibody. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
The FTA-ABS test (Fluorescent Treponemal Antibody Absorption Test) for syphilis is an example of an indirect fluorescent antibody procedure. This is a confirming test for syphilis since it tests specifically for antibodies in the patient's serum made in response to the syphilis spirochete, Treponema pallidum.
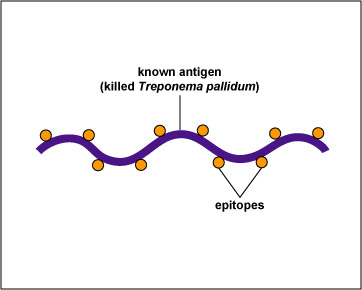
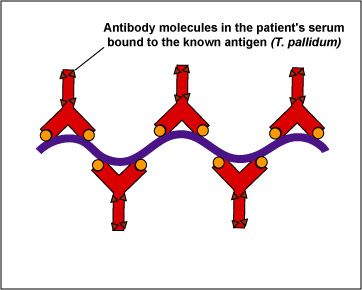
In this test, killed T. pallidum,(the known antigen), is fixed on a slide (see Fig 14, step 1). The patient's serum is added to the slide. If the patient has syphilis, antibodies against the T. pallidum will react with the antigen on the slide (Fig. 14, step 2). The slide is then washed to remove any antibodies not bound to the spirochete.
Fig. 14, Step 1
FTA Test for Antibodies Against Treponema pallidum
Fig. 14, Step 2
FTA Test for Antibodies Against Treponema pallidumTreponema pallidum, the known antigen, is fixed to a microscope slide. If there are antibodies against Treponema pallidum in the patient's serum, they will bind to the spirochete. All other antibodies are washed from the slide. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
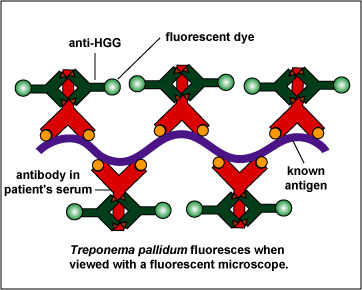
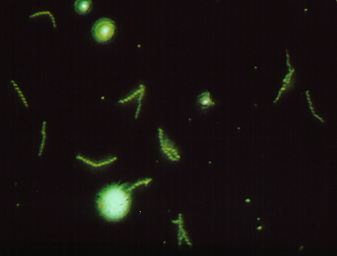
To make this reaction visible, a second animal-derived antibody made against human antibodies and labelled with a fluorescent dye (fluorescent anti-human gamma globulin) is added. These fluorescent anti-HGG antibodies react with the patient's antibodies which have reacted with the T. pallidum on the slide (Fig. 14, step 3). The slide is washed to remove any unbound fluorescent anti-HGG antibodies and observed with a fluorescent microscope. If the spirochetes glow or fluoresce (see Fig. 15), the patient has made antibodies against T. pallidum and has syphilis.
Fig. 14, Step 3
FTA Test for Antibodies Against Treponema pallidumFig. 15: A Positive FTA Test for Syphilis Viewed with a Flourescent Microscope
Fluorescent anti-human gamma globulin (anti-HGG) is added to the well. (Anti-HGG is an antibody made by another animal against human IgG antibodies. A fluorescent dye is then attached to the antibody.) The anti-HGG will with any human IgG antibodies bound to the Treponema pallidum on the slide. All unbound anti-HGG is then washed from the slide. When viewed with a flourescent microscope, the spirochetes will fluoresce. Note the fluorescing spirochetes. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
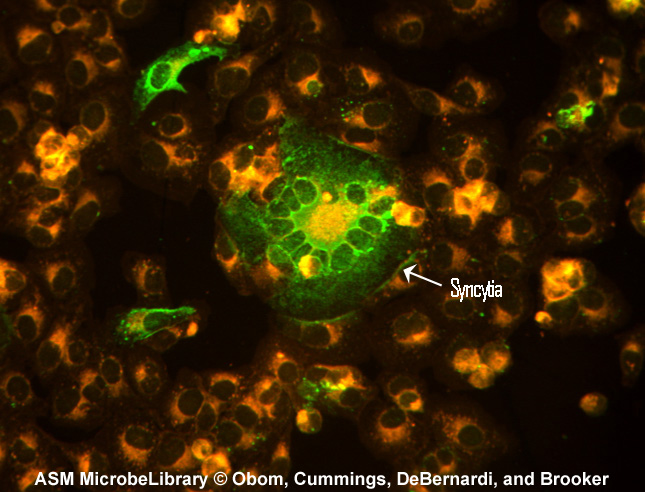
Another example of the indirect fluorescent antibody test is the test for antibodies against the measles virus. Inactivated measles virus-infected cells (the known antigen) are fixed to a microscope slide. The patient's serum is then added. If the person has measles, antibodies of the isotype IgG will be made against the measles virus and will bind to viral epitopes on the know measles virus-infected cells. After washing the slide to remove any unbound IgG, fluorescent antihuman IgG is added. The fluorescent antihuman IgG then binds to the patient's IgG that is bound to the infected cells. When viewed with a fluorescent microscope, the infected cells will fluoresce green as seen in Fig. 16.
Fig. 16: Indirect Fluorescent Antibody Test for Measles Antibody
Another example of the indirect fluorescent antibody test is the test for antibodies against the measles virus. Inactivated measles virus-infected cells (the known antigen) are fixed to a microscope slide. The patient's serum is then added. If the person has measles, antibodies of the isotype IgG will be made against the measles virus and will bind to viral epitopes on the know measles virus-infected cells. After washing the slide to remove any unbound IgG, fluorescent antihuman IgG is added. The fluoprescent antihuman IgG then binds to the patient's IgG that is bound to the infected cells. When viewed with a fluorescent microscope, the infected cells will fluoresce green. Syncytia are fused virus-infected cells. Indirect Immunofluorescence for Detection of Measles Antibody.
Kristina M. Obom, Patrick J. Cummings, Maria A. DeBernardi, Gary Brooker, authors.
Licensed for use, ASM MicrobeLibrary.
e. The EIA and Western Blot serologic tests for antibodies against the Human Immunodeficiency Virus (HIV)
In the case of the current HIV antibody tests, the patient's serum is mixed with various HIV antigens produced by recombinant DNA technology. If the person is seropositive (has repeated positive antigen-antibody tests), then HIV must be in that person's body stimulating antibody production. In other words, the person must be infected with HIV. The two most common tests currently used to detect antibodies against HIV are the enzyme immunoassay or EIA (also known as the enzyme-linked immunosorbent assay or ELISA) and the Western blot or WB. A person is considered seropositive for HIV infection only after an EIA screening test is repeatedly reactive and another test such as the WB has been performed to confirm the results.
The EIA is less expensive, faster, and technically less complicated than the WB and is the procedure initially done as a screening test for HIV infection. The various EIA tests give a spectrophotometric reading of the amount of antibody binding to known HIV antigens.
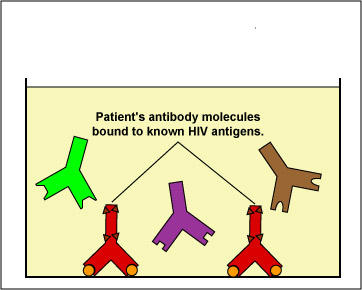
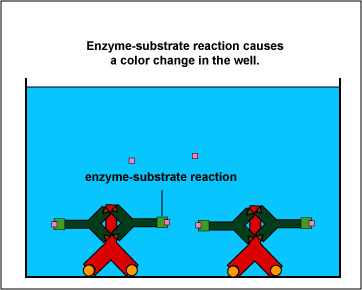
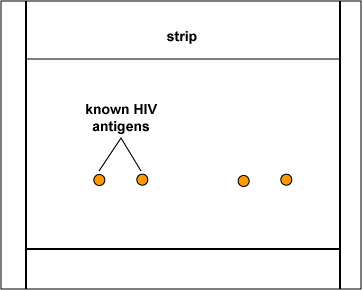
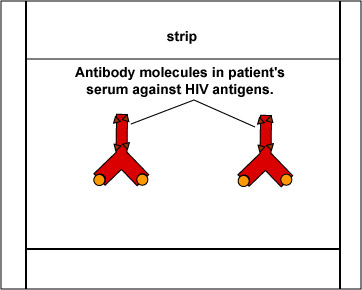
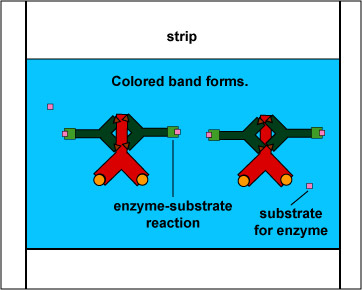
The EIA test kit contains plastic wells to which various HIV antigens have been adsorbed (see Fig. 17, step 1). The patient's serum is added to the wells and any antibodies present in the serum against HIV antigens will bind to the corresponding antigens in the wells (Fig. 17, step 2). The wells are then washed to remove all antibodies in the serum other than those bound to HIV antigens. Enzyme linked anti-human gamma globulin (anti HGG) antibodies are then added to the wells. These antibodies, made in another animal against the Fc portion of human antibodies by injecting the animal with human serum, have an enzyme chemically attached. They react with the human antibodies bound to the known HIV antigens (Fig. 17, step 3). The wells are then washed to remove any anti-HGG that has not bound to serum antibodies. A substrate specific for the enzyme is then added and the resulting enzyme-substrate reaction causes a color change in the wells (Fig. 17, step 4). If there are no antibodies in the patient's serum against HIV, there will be nothing for the enzyme-linked anti-HGG to bind to and it will be washed from the wells. When the substrate is added, there will be no enzyme present in the wells to give a color change.
Fig. 17, Step 1
Enzyme Immunoassay (EIA or ELISA) for HIV AntibodiesFig. 17, Step 2
Enzyme Immunoassay (EIA or ELISA) for HIV AntibodiesKnown HIV antigens are adsorbed to test well. The patient's serum is added. If the serum contains antibodies against the known HIV antigens, they will bind to those antigens. All other antibodies are then washed from the well. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Fig. 17, Step 3
Enzyme Immunoassay (EIA or ELISA) for HIV AntibodiesFig. 17, Step 4
Enzyme Immunoassay (EIA or ELISA) for HIV AntibodiesEnzyme-linked anti-human gamma globulin (anti-HGG) is added to the well. (Anti-HGG is an antibody made by another animal against human IgG antibodies. An enzyme is then attached to the antibody.) The anti-HGG will with any human IgG antibodies bound to the adsorbed HIV antigens. All unbound anti-HGG is then washed from the well. The substrate for the enzyme attached to the anti-HGG is added to the well. The enzyme substrate reaction produces a visible color change which can be measured with a spectrophotometer. This shows that the patient's serum must have contained antibodies against the known HIV antigens. If there were no antibodies present then there would be no enzyme-linked anti-HGG in the well and no color-producing enzyme-substrate reaction. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
If the initial EIA is reactive, it is automatically repeated to reduce the possibility that technical laboratory error caused the reactive result. If the EIA is still reactive, it is then confirmed by the Western blot test.
The Western blot WB is the test most commonly used as a confirming test if the EIA is repeatedly positive. The WB is technically more complex to perform and interpret, is more time consuming, and is more expensive than the EIAs.
With the WB, the various protein and glycoprotein antigens from HIV are separated according to their molecular weight by gel electrophoresis (a procedure that separates charged proteins in a gel by applying an electric field). Once separated, the various HIV antigens are transferred to a nitrocellulose strip (see Fig. 18, step 1 and Fig. 18, step 2). The patient's serum is then incubated with the strip and any HIV antibodies that are present will bind to the corresponding known HIV antigens on the strip (Fig. 18, step 3). Enzyme-linked anti-human gamma globulin (anti HGG) antibodies are then added to the strip. These antibodies, made in another animal against the Fc portion of human antibodies by injecting the animal with human serum, have an enzyme chemically attached. They react with the human antibodies bound to the known HIV antigens (Fig. 18, step 4). The strip is then washed to remove any anti-HGG that has not bound to serum antibodies. A substrate specific for the enzyme is then added and the resulting enzyme-substrate reaction causes a color change on the strip (Fig. 18, step 5). If there are no antibodies in the patient's serum against HIV, there will be nothing for the enzyme-linked anti-HGG to bind to and it will be washed from the strip. When the substrate is added, there will be no enzyme present on the strip to give a color change.
Fig. 18, Step 1: Western Blot (WB) Test for HIV Antibodies
Fig. 18, Step 2: Western Blot (WB) Test for HIV Antibodies
Fig. 18, Step 3: Western Blot (WB) Test for HIV Antibodies
Different known HIV antigens are separated on the WB test strip. Section of the strip with known HIV antigen gp120. The patient's serum is added. If the serum contains antibodies against any of the known HIV antigens, they will bind to those antigens on the strip. All other antibodies are then washed from the strip. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Fig. 18, Step 4: Western Blot (WB) Test for HIV Antibodies
Fig. 18, Step 5: Western Blot (WB) Test for HIV Antibodies
Enzyme-linked anti-human gamma globulin (anti-HGG) is added to the well. (Anti-HGG is an antibody made by another animal against human IgG antibodies. An enzyme is then attached to the antibody.) The anti-HGG will with any human IgG antibodies bound to the adsorbed HIV antigens. All unbound anti-HGG is then washed from the strip. The substrate for the enzyme attached to the anti-HGG is added to the strip. The enzyme substrate reaction produces a visible color change. This shows that the patient's serum must have contained antibodies against the known HIV antigens on the strip where the reaction took place. If there were no antibodies present then there would be no enzyme-linked anti-HGG in the well and no color-producing enzyme-substrate reaction.
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
It should be mentioned that all serologic tests give occasional false-positive and false-negative results. The most common cause of a false-negative HIV antibody test is when a person has been only recently infected with HIV and his or her body has not yet made sufficient quantities of antibodies to give a visible positive serologic test. It generally takes between 2 weeks and 3 months after a person is initially infected with HIV to convert to a positive HIV antibody test.
For more information on HIV and AIDS, see the following Learning Object in my CourseArc lessons:
f. Diagnosis of COVID-19 infection by the detection of antibodies made against SARS-CoV-2 antigensMost of these tests look for the presence of antibodies (often IgM made early during an infection, as well as IgG made later during an infection and for a longer duration) against the N (nuclear) antigen of SARS-CoV-2. There is a greater problem with false-positive and false-negative results compared to the tests that detect SARS-CoV-2 RNA but test results can be determined much more quickly. Antibody tests are not currently recommend as the sole basis for diagnosis of acute COVID-19 infection.
For more information on SARS-CoV-2 and Covid 19, see the following Learning Object in my CourseArc lessons:
4. Sensitivity and Specificity of Serologic Testing
Sensitivity measures the "true-positive" rate of the serologic test, that is, how often a test correctly generates a positive result for people who have the disease being tested. To give an example, a test having a 95% sensitivity will correctly give a positive result for 95% of the people who have that disease, but will give a negative result, or false-negative, for 5% of the people who have the disease and should have tested positive. So, a high-sensitivity serologic test will correctly identify almost everyone who does have the disease and will generate few false-negative results.
Specificity measures the "true-negative" rate of the serologic test, that is, how often a test correctly generates a negative result for people who do not have the disease being tested. As an example, a test having a 95% specificity will correctly give a negative result for 95% of people who do not have the disease, but will return a positive result, or false-positive, for 5% of the people who don't have the disease and should have tested negative. So, a high-specificity serologic test will correctly rule out almost everyone who does not have the disease and will generate few false-positive results.
However, no serologic tests are 100% accurate. When considering test accuracy, the rate of infection also needs to be considered. In a region with a low disease prevalence, the risk of false positive results by serologic testing is higher, even with excellent specificity. However, in a region with a high disease prevalence, the risk of false negative results will be higher, even with excellent sensitivity.
Medscape articles on infections associated with organisms/diseases mentioned
in this lab exercise. Registration to access this website is free. PROCEDURE FOR DIRECT SEROLOGIC TESTING TO DETECT UNKNOWN ANTIGENS
A. Serologic Typing of Shigella
1. Using a wax marker, draw two
circles (about the size of a nickel) on each of two clean glass slides
. Label the circles A, B, C, and D. 2. Add one drop of the
suspected Shigella (unknown antigen) to each circle. (The Shigella
has been treated with formalin to make it noninfectious but still antigenic.)
3. Now add one drop of
known Shigella subgroup A antiserum to the "A" circle, one drop
of known Shigella subgroup B antiserum to the "B" circle,
one drop of known Shigella subgroup C antiserum to the
"C" circle, and one drop of known Shigella subgroup D
antiserum to the "D" circle. 4. Rotate the slide carefully
for 30-60 seconds. Agglutination
of the bacteria, indicates a positive reaction. No agglutination
is negative. 5. Dispose of all pipettes and
slides in the disinfectant container. B. Serologic Typing of Streptococci: The Clearview® Strep A Exact II Dipstick
Test 1. Add 4 drops of Extraction
Reagent #1 to the extraction tube. This reagent contains 2M sodium
nitrite and should be pink to purple in color. 2. Add 4 drops of Extraction
Reagent #2 to the extraction tube. This reagent contains 0.3M acetic
acid. The solution must turn yellow in color. 3. Place the throat swab
in the extraction tube and roll it with a circular motion inside the tube.
Let stand for at least 1 minute. 4. Squeeze the swab firmly against
the extraction tube to expel as much liquid as possible from the swab and
discard the swab in the biowaste container. 5. Immerse the test strip
into the extraction tube with the arrows pointing toward the extracted
sample solution. Leave the strip in the tube and start timing. 6. Read results in 5 minutes.
A red band in the control region and a red band in the test region indicates
a positive test. A
single red band in the control region only indicates a negative test. No colored band in the control region
indicates an invalid test. C. Serologic Testing to Detect
Pregnancy: The Alere hCG-Dipstick® 1. Dip the hCG dipstick into the urine up to the maximum line on the strip for 5 seconds. 2. Place the test dipstick on a flat, non-absorbant surface and read the results at 3-4 minutes. Do not interpret after the appropriate read time. 3. If hCG is present in the urine at a concentration of 25mlU/ml or greater, a positive test, a pink-to-red Test line will appear along with a red Control line in the Result Window. If hCG is absent or present at very low levels, a negative test, only a red Control line appears in the Result Window. D. The Direct Fluorescent
Antibody Technique
Observe the demonstration of a
positive direct fluorescent antibody test for Neisseria
gonorrhoeae . PROCEDURE FOR INDIRECT SEROLOGIC TESTING TO DETECT ANTIBODIES IN THE PATIENT'S SERUM A. The RPR® Card Test for Syphilis (demonstration)
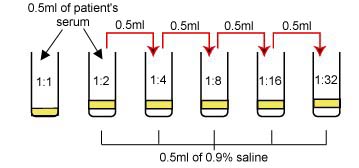
1. Label 6 test tubes as follows:
1:1, 1:2, 1:4, 1:8, 1:16, and 1:32. 2. Using a 1.0 ml pipette, add 0.5 ml of 0.9% saline solution into tubes 1:2, 1:4, 1:8, 1:16, and
1:32. 3. Add 0.5 ml of the patient's
serum to the 1:1 tube (undiluted serum). 4. Add another 0.5 ml of serum to the saline in the 1:2 tube and mix. Remove 0.5 ml from the
1:2 tube and add it to the 1:4 tube and mix. Remove 0.5 ml from
the 1:4 tube, add to the 1:8 tube and mix. Remove 0.5 ml from
the 1:8 tube, add to the 1:16 tube and mix. Remove 0.5 ml from
the 1:16 tube, add to the 1:32 tube and mix. Remove 0.5 ml from
the 1:32 tube and discard. The dilution of the serum is summarized
in Fig. 19.
Gary E. Kaiser, Ph.D. 5. Using the capillary pipettes
provided with the kit, add a drop of each serum dilution to separate
circles of the RPR card. Spread the serum over the entire inner surface of
the circle with the tip of the pipette, using a new pipette for each serum
dilution. 6. Using the RPR antigen dispenser,
add a drop of known RPR antigen to each circle. Do not let the needle
of the dispenser touch the serum. Using disposable stirrers, mix the known
RPR antigen with the serum in each circle. 7. Place the slide on a shaker
and rotate for a maximum of 4 minutes. 8. Read the results as follows: The greatest serum dilution that
produces a reactive result is the titer. For example, if the
dilutions turned out as follows, the titer would be reported as 1:4 or 4 dils. B. The Serologic Tests for Infectious
Mononucleosis (demonstration)
1. Place one drop of each of
the patient's serum in circles on the test slide. 2. Add one drop of thedyed, color-enhanced horse erythrocytes containing Paul-Bunnell antigens (the known antigen) to each circle next to the patient's serum and mix with the disposable
applicator sticks. 3. Rock the card gently for 2 minutes,and observe
for agglutination of the horse erythrocytes. Agglutination of the red bllod cells indicates the presence
of heterophile antibodies. C. The Serologic Tests for Systemic
Lupus Erythematosus (SLE) (demonstration)
1. Add one drop of each of
the patient's serum to separate circles on the test slide. 2. Add one drop of the Latex-Deoxyribonucleoprotein
reagent (the known antigen, deoxyribonucleoprotein adsorbed to latex particles)
to each serum sample and mix with disposable applicator sticks. 3. Rock the slide gently for 3 minutes and look for agglutination of the latex particles. Agglutination of the latex particles indicates
the presence of antinuclear antibodies associated with SLE. D. The FTA-ABS Test for Syphilis
(Indirect Fluorescent Antibody Technique
Observe the 35mm slide of a positive
FTA-ABS test. E. The EIA and WB Tests for HIV
Antibodies
Observe the illustrations of the EIA test (shown in Fig. 17, step 1 through Fig. 17, step 4 above) and the WB test (shown in Fig. 18, step 1 through Fig. 18, step 5 above) to detect antibodies
against HIV antigens. RESULTS FOR DIRECT SEROLOGIC TESTING TO DETECT UNKNOWN ANTIGENS A. Serologic Typing of Shigella
Make a drawing of your results.
B. Serologic Typing
of Streptococci: Clearview® Strep A Exact II Dipstick Make a drawing of your results.
C. Serologic Testing
to Diagnose Pregnancy: Alere® hCG Dipstick Make a drawing of a positive test for pregnancy. D. The Direct Fluorescent
Antibody Technique Make a drawing and describe a
positive direct fluorescent antibody test. RESULTS FOR INDIRECT SEROLOGIC TESTING TO DETECT ANTIBODIES IN THE PATIENT'S SERUM A. RPR Card® Test for Syphilis
(Quantitative) Detects nontreponemal antilipid
antibodies (reagin) Record your results in the table.
R
= reactive (distinct clumps) B. MONO-TEST for Infectious Mononucleosis
(Qualitative)
Detects heterophile antibodies. Draw the results of a positive
and a negative test. Infectious
mononucleosis test slide
C. Serologic test
for SLE (Qualitative)
Detects anti-deoxyribonucleoprotein
antibodies. Draw the results of a positive
and a negative test. SLE test slide
D. FTA-ABS Test for Syphilis (Confirming)
Detects antibodies against Treponema
pallidum Draw the results of a positive
FTA-ABS test. After completing
this lab, the student will be able to perform the following objectives:
A. INTRODUCTION TO SEROLOGICAL TESTING
1. Define serology. 2. Define antigen and state what
may act as an antigen. 3. Define antibody and state where
they are primarily found in the body. 4. Define direct serologic testing
and indirect serologic testing. B. DIRECT SEROLOGIC TESTING: USING ANTIGEN-ANTIBODY
REACTIONS IN THE LAB TO IDENTIFY UNKNOWN ANTIGENS SUCH AS MICROORGANISMS
Discussion 1. Define antiserum. 2. Describe two ways of producing
known antiserum. 3. Describe the concept and general
procedure for using serologic testing to identify unknown antigens (direct
serologic testing). 5. Describe how to serologically
identify Lancefield group A Streptococcus causing pharyngitis using the Rapid Response Strep A
Test. 6. Describe how to diagnose pregnancy
serologically using the QuickVue+® One-Step hCG-Combo Test. 7. Briefly describe the direct
fluorescent antibody technique. Results
1. Correctly interpret the results
of the following serological tests: a. serological typing of Shigella
b. serological identification
of Group A streptococcal antigen using the Rapid Response Strep A
Test. c. serological testing for pregnancy
using the QuickVue+® One-Step hCG-Combo Test d. a direct fluorescent antibody
test C. INDIRECT SEROLOGIC TESTING: USING ANTIGEN-ANTIBODY
REACTIONS IN THE LAB TO DETECT ANTIBODIES IN THE PATIENT's SERUM Discussion 1. State the principle and the
general procedure behind indirect serologic testing. 2. State the difference between
a qualitative serological test and a quantitative serological test. 3. Define titer. 4. State what disease the RPR
and the FTA-ABS procedures test for. Indicate which of these is a presumptive
test, which is a confirming test, and why. 5. State the significance of non-treponemal
anti-lipid (reagin) antibodies in serological testing. 6. State the significance of heterophile
antibodies in serological testing. 7. State the significance of anti-deoxyribonucleoprotein
antibodies in serological testing. 8. Briefly describe the indirect
fluorescent antibody technique. 9. Briefly describe the EIA test
for HIV antibodies and state the significance of a positive HIV antibody test. 10. In terms of serologic testing, define sensitivity and specificity. 11. State how the prevalence of a disease in a region effects the number of false-positive or false-negatives in the population of that region, even with tests of high sensitivity and specificity. Results
1. Interpret the results of the
following serological tests: a. serologic test for infectious
mononucleosis 2. Determine the titer of a quantitative
RPR Card® test.
SELF-QUIZ
Fig. 19: Diluting the Patient's Serum in a Quantitative RPR Card Test
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License

1:1
1:2
1:4
1:8
1:16
1:32
Dilution
Result
1:1
1:2
1:4
1:8
1:16
1:32
Titer
N = nonreactive (no clumps)
+ =
Agglutination of the horse erythrocytes
- = No agglutination of the horse erythrocytes

+ = Agglutination
- = No agglutination
PERFORMANCE
OBJECTIVES FOR LAB 16
b. serologic test for
SLE
c. FTA-ABS test

Microbiology Laboratory Manual by Gary E. Kaiser, PhD, Professor of Microbiology
is licensed under a Creative Commons Attribution 4.0 International License.
Last updated: May, 2023