A. 1 ml of bacteria is mixed with 1 ml of sterile saline. The total ml in the tube would be 2 ml, of which 1 ml is bacteria. This is a 1:2 dilution (also written 1/2, meaning 1/2 as many bacteria per ml as the original ml).
B. 1 ml of bacteria is mixed with 3 ml of sterile saline. The total ml in the tube would be 4 ml, of which 1 ml is bacteria. This is then a 1:4 dilution (also written 1/4, meaning 1/4 as many bacteria per ml as the original ml).
C. 1 ml of bacteria is mixed with 9 ml of sterile saline. The total ml in the tube would be 10 ml, of which 1 ml is bacteria. This is then a 1:10 dilution (also written 1/10 or 10-1, meaning 1/10 or 10-1 as many bacteria per ml as the original ml).
D. For dilutions greater than 1:10, usually serial dilutions (dilutions of dilutions) are made. The following represents a serial ten-fold dilution ( a series of 1:10 dilutions):
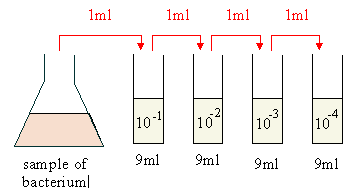 |
- The dilution in tube #1 would be 1/10 or 10-1.
- The dilution in tube #2 would be 1/100 or 10-2 (1/10 of 1/10).
- The dilution in tube #3 would be 1/1000 or 10-3 (1/10 of 1/100).
- The dilution in tube #4 would be 1/10,000 or 10-4 (1/10 of 1/1000).
