There are two genera of bacteria that can appear as a streptococcus arrangement that we will take up in the lab: the genus Streptococcus (see Fig. 1) and the genus Enterococcus (see Fig. 2). Both are Gram-positive cocci 0.5-1.0 µm in diameter, typically occurring in pairs and chains of varying length when grown in a liquid medium, and often occurring singly, in pairs, short chains, and clusters when taken from an agar culture. As learned in Lab 8, they are both catalase-negative.
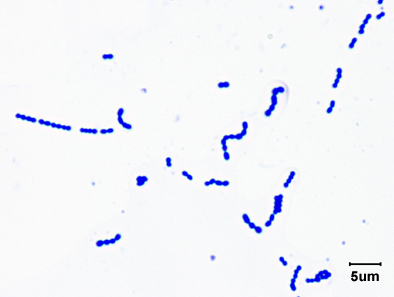
Fig. 1: Gram Stain of Streptococcus pyogenes
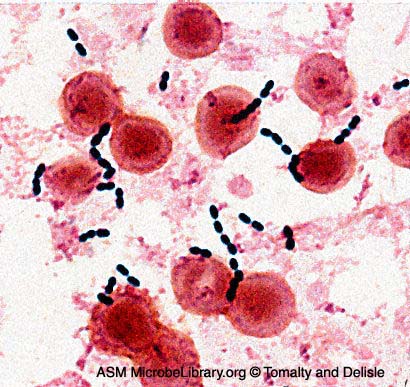
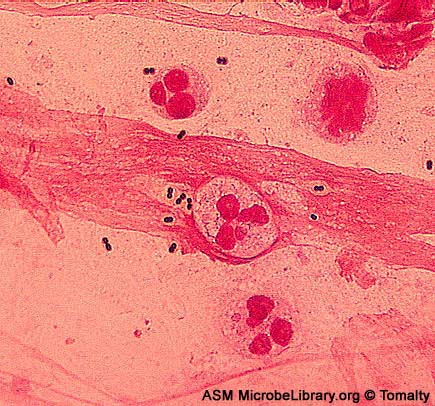
Fig. 2: Gram Stain of Enterococcus faecalis in a Blood Culture
Note Gram-positive (purple) cocci in chains (arrows). Streptococcus pyogenes is the species of Streptococcus responsible for strep throat. Enterococcus species are normal glora of the intestinal tract. Enterococcus species frequently causes infections within the peritoneal cavity, especially following penetrating trauma such as gunshot wounds, knife wounds, and surgical wounds, urinary tract infections, kidney infections, prostate infections, and infections of damaged or compromised skin, such as diabetic or decubitus ulcers, burns, and surgical wounds. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Image: Enterococcus faecalis in a Blood Culture. © Gloria Delisle and Lewis Tomalty, authors.
Licensed for use, ASM MicrobeLibrary.
A. The genus Streptococcus
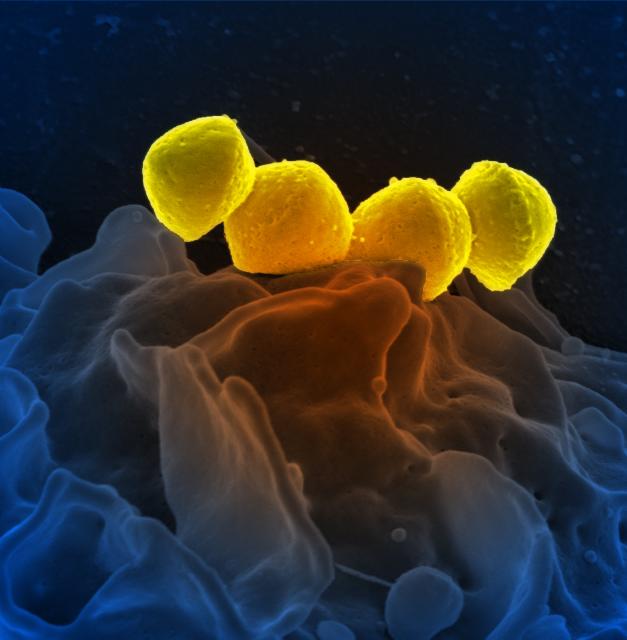
Streptococcus species are usually classified clinically based on their hemolytic properties on blood agar and according to their serologic groups. A scanning electron micrograph of Streptococcus pyogenes is shown in Fig. 3 and a scanning electron micrograph of Streptococcus pneumoniae is shown in Fig. 4.
Fig. 3: Scanning Electron Micrograph of Streptococcus pyogenes Fig. 4: Scanning Electron Micrograph of Streptococcus pneumoniae
By National Institutes of Health (NIH) (National Institutes of Health (NIH)) [Public domain], via Wikimedia Commons By Content Providers(s): CDC/ Janice Haney Carr [Public domain]
Courtesy of the Centers for Disease Control and Prevention.
The streptococci are usually isolated on Blood agar. Blood agar is one of the most used media in a clinical lab. It consists of an enriched agar base (Tryptic Soy agar) to which 5% sheep red blood cells have been added. Blood agar is commonly used to isolate not only streptococci, but also staphylococci and many other pathogens. Besides providing enrichments for the growth of fastidious pathogens, Blood agar can be used to detect hemolytic properties.
Hemolysis refers to is the lysis of the red blood cells in the agar surrounding bacterial colonies and is a result of bacterial enzymes called hemolysins. Although hemolysis can often be observed with the naked eye, ideally it should be examined microscopically using low power magnification, especially in cases of doubtful hemolysis. Reactions on blood agar are said to be beta, alpha, gamma, or double-zone:
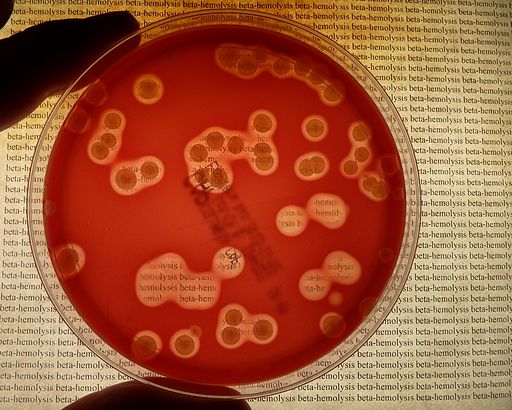
1. Beta hemolysis (see Fig. 5A, Fig. 5B, and Fig. 5C) refers to a clear, red blood cell-free zone surrounding the colony, where a complete lysis of the red blood cells by the bacterial hemolysins has occurred. This is best seen in subsurface colonies where the agar has been stabbed since some bacterial hemolysins, like streptolysin O, are inactivated by oxygen.
Fig. 5A: Beta Hemolysis on Blood Agar (Indirect Lighting)
Fig. 5B: Beta Hemolysis on Blood Agar (Indirect Lighting)
Fig. 5C: Beta Hemolysis on Blood Agar)
> Note clear, colorless zone surrounding colonies where complete lysis of the red blood cells by the hemolysins has occurred. Note clear, colorless zone surrounding colonies where complete lysis of the red blood cells by the hemolysins has occurred. You can actually read text through an area of beta hemolysis. Note clear, colorless zone surrounding colonies where complete lysis of the red blood cells by the hemolysins has occurred. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
By HansN. (Own work) [CC BY-SA 3.0 (https://creativecommons.org/licenses/by-sa/3.0)], via Wikimedia Commons. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
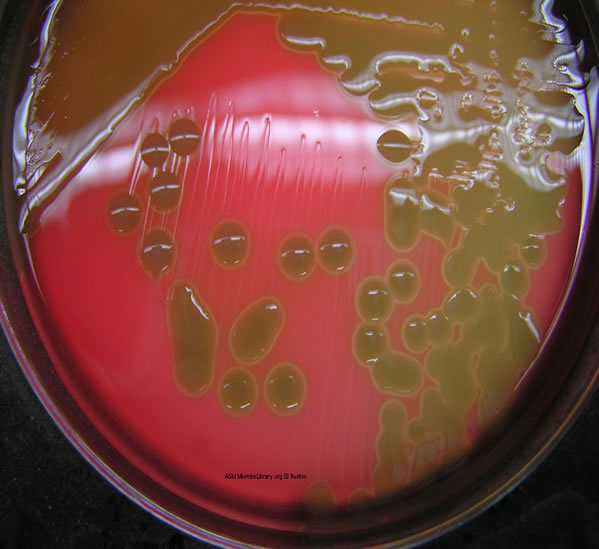
2. Alpha hemolysis (see Fig. 6A and Fig. 6B) appears as a zone of partial hemolysis surrounding the colony, often accompanied by a greenish discoloration of the agar. This is also best seen in subsurface colonies where the agar has been stabbed.
Fig. 6A: Alpha Hemolysis on Blood Agar (Indirect Lighting)
Fig. 6B: Streptococcus pneumoniae on Blood Agar Showing Alpha Hemolysis
Note the partial hemolysis accompanied by a greenish discolorization of the agar around the growth. Note the mucoid, transluscent colonies and the alpha hemolysis (partial hemolysis typically accompanied by a greenish discolorization of the agar around and under the growth). Photograph from From MicrobeLibrary.org
Courtesy of Rebecca Buxton, University of UtahPhotograph from MicrobeLibrary.org
Courtesy of Rebecca Buxton, University of Utah
3. Gamma reaction (see Fig. 7) refers to no hemolysis or discoloration of the agar surrounding the colony.
Fig. 7: Gamma Reaction on Blood Agar
Note there is no hemolysis and no change in the blood agar.Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
4. Double-zone hemolysis refers to both a beta and an alpha zone of hemolysis surrounding the colony.
See Fig. 8 to view a photograph showing alpha, beta, and gamma hemolysis on blood agar.
See Fig. 9 for a blood agar plate of a throat culture showing possible Streptococcus pyogenes.
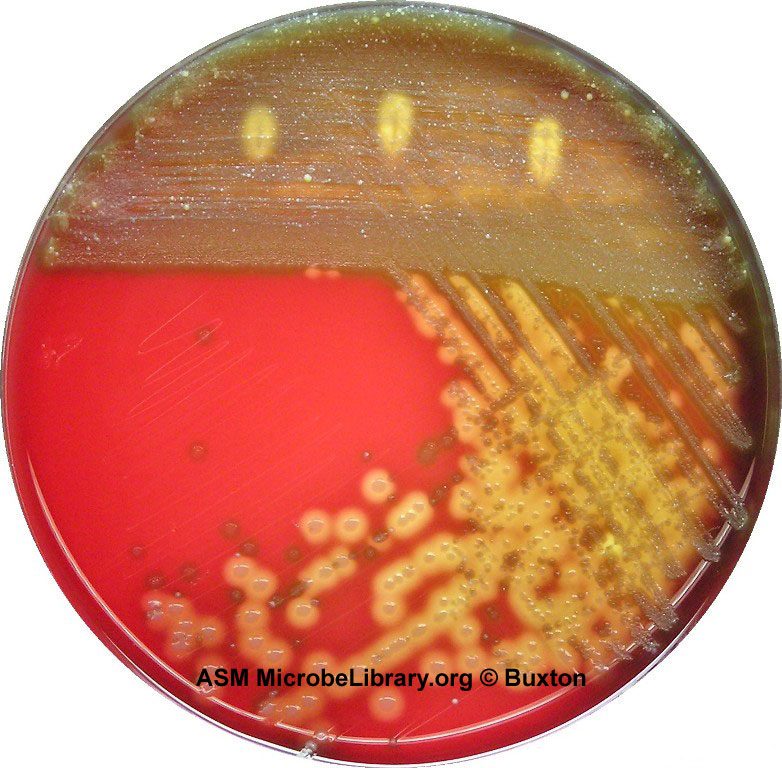
Fig. 8: A Plate of Blood Agar Showing Alpha, Beta, and Gamma Hemolysis (Indirect Lighting)
Fig. 9: A Blood Agar Plate of a Throat Culture Showing Possible Streptococcus pyogenes.
Alpha-, beta-, and gamma-hemolytic bacteria were streaked to form of the Greek letters alpha, beta, and gamma. Note beta colonies mixed in with the normal alpha and gamma colonies of the viridans streptococci that normally inhabit the throat. By Y tambe (Y tambe's file) [GFDL (http://www.gnu.org/copyleft/fdl.html), CC-BY-SA-3.0 (http://creativecommons.org/licenses/by-sa/3.0/) or CC BY-SA 2.5-2.0-1.0 (https://creativecommons.org/licenses/by-sa/2.5-2.0-1.0)], via Wikimedia Commons Photograph from MicrobeLibrary.org
Courtesy of Rebecca Buxton, University of Utah
Many of the streptococci can also be classified under the Lancefield system. In this case, they are divided into 19 different serologic groups on the basis of carbohydrate antigens in their cell wall. These antigenic groups are designated by the letters A to H, K to M, and O to V. Lancefield serologic groups A, B, C, D, F, and G are the ones that normally infect humans, however, not all pathogenic streptococci can be identified by Lancefield typing (e.g., Streptococcus pneumoniae). Serologic typing to identify microorganisms will be discussed in more detail later in Lab 16. Single-stranded DNA probes complementary to species-specific r-RNA sequences of streptococci and enterococci are also being used now to identify these organisms.
1. The Beta Streptococci
DISCUSSION
Lancefield serologic groups A, B, C, D, F, and G are all streptococci that may show beta hemolysis on Blood agar. However, some group B streptococci are non-hemolytic and group D streptococci (discussed below) usually show alpha hemolysis or are non-hemolytic.
Streptococcus pyogenes, often referred to as group A beta streptococci or GAS because they belong to Lancefield serologic group A and show beta hemolysis on blood agar, are responsible for most acute human streptococcal infections. S. pyogenes isolates are Gram-positive cocci 0.5-1.0 µm in diameter that typically form short chains in clinical specimens and longer chains in laboratory media. The most common infection is pharyngitis (streptococcal sore throat) with the organism usually being limited to the mucous membranes and lymphatic tissue of the upper respiratory tract. S. pyogenes is responsible for 15-30% of cases of acute pharyngitis in children and 5-10% of cases in adults. Between 5% and 20% of children are asymptomatic carriers. Pharyngitis is spread person to person primarily by respiratory droplets; skin infections are spread by direct contact with an infected person or through fomites. S. pyogenes produces a hyaluronic acid capsule which is chemically like host connective tissue and masks the bacteria from immune recognition as well as enabling the bacteria to resist phagocytosis. Another characteristic of S. pyogenes is the organism’s ability to invade epithelial cells. This may play a role in some people becoming carriers of S. pyogenes and the bacteria not being eradicated by antibiotics.
From the pharynx, however, the streptococci sometimes spread to other areas of the respiratory tract resulting in laryngitis, bronchitis, pneumonia, and otitis media (ear infection). Occasionally, it may enter the lymphatic vessels or the blood and disseminate to other areas of the body, causing septicemia, osteomyelitis, endocarditis, septic arthritis, and meningitis. It may also infect the skin, causing erysipelas, impetigo, or cellulitis.
Group A beta streptococcus infections can result in two autoimmune diseases, rheumatic fever and acute glomerulonephritis, where antibodies made against streptococcal antigens cross react with joint membranes and heart valve tissue in the case of rheumatic fever, or glomerular cells and basement membranes of the kidneys in the case of acute glomerulonephritis.
Streptococcal pyrogenic exotoxin (Spe), produced by rare invasive strains and scarlet fever strains of Streptococcus pyogenes (the group A beta streptococci). S. pyogenes produces a number of SPEs that are cytotoxic, pyrogenic, enhance the lethal effects of endotoxins, and contribute to cytokine-induced inflammatory damage. SPEs are responsible for causing streptococcal toxic shock syndrome (STSS) whereby excessive cytokine production leads to fever, rash, and triggering the shock cascade. The SPEs also appear to be responsible for inducing necrotizing fasciitis, a disease that can destroy the skin, fat, and tissue covering the muscle (the fascia). SPE B is also a precursor for a cysteine protease that can destroy muscles tissue.
CDC reports that approximately 9,000-11,500 cases of invasive GAS disease occur each year in the U.S., with STSS and necrotizing fasciitis each accounted for approximately 6-7% of the cases. STSS has a mortality rate of around 35%. The mortality rate for necrotizing fasciitis is approximately 25%.
For further information on virulence factors for group A beta Streptococci, see the following Softchalk lessons:
- Gram-Positive PAMPs
- The Ability to Adhere to Host Cells
- The Ability to Resist Phagocytic Engulfment
- The Ability to Resist Phagocytic Destruction
- The Ability to Evade Adaptive Immune Defenses
- Type I Toxins
- Type II Toxins that Damage Cell Membranes
- The Ability to Induce Autoimmune Responses
The group B streptococci (GBS or Streptococcus agalactiae) usually show a small zone of beta hemolysis on Blood agar, although some strains are non-hemolytic. S. agalactiae isolates are Gram-positive cocci 0.6-1.2 µm in diameter that typically form short chains in clinical specimens and longer chains in laboratory media. They are found in the gastrointestinal tract and genitourinary tract of 15%-45% healthy woman. This reservoir, along with nosocomial transmission, provides the inoculum by which many infants are colonized at birth. The transmission rate from a mother colonized with GBS to her baby is thought to be around 50%. Most colonized infants (and adults) remain asymptomatic, however, an estimated 1-2% of neonates colonized will develop invasive GBS diseases, including pneumonia, septicemia, and/or meningitis. Pregnant women should be tested to determine if they are GBS carriers and be given IV antibiotics if they are a carrier
Other infections associated with group B streptococci include urinary tract infections, skin and soft tissue infections, osteomyelitis, endometritis, and infected ulcers (decubitus ulcers and ulcers associated with diabetes). In the immunocompromised patient it sometimes causes pneumonia and meningitis.
The group C streptococci (mainly S. equi, S. equisimilis and S. zooepidemicus) are beta hemolytic. They sometimes cause pharyngitis and, occasionally, bacteremia, endocarditis, meningitis, pneumonia, septic arthritis, and cellulitis. Group C streptococci are a common cause of infections in animals.
The group F streptococci (mainly S. anginosus) have been isolated from abscesses of the brain, mouth, and jaw. They also sometimes cause endocarditis.
The group G streptococci also show beta hemolysis. They sometimes cause pharyngitis and can also cause serious infections of the skin and soft tissues (mainly in the compromised host) as well as endocarditis, bacteremia, and peritonitis.
All of these beta hemolytic streptococci can be identified by biochemical testing and/or by serologic testing. Today you will look at the isolation and identification of group A beta streptococci (Streptococcus pyogenes) by biochemical testing. Serological identification will be performed in Lab 16.
ISOLATION AND IDENTIFICATION OF
GROUP A BETA STREPTOCOCCI (Streptococcus pyogenes)
Videos reviewing techniques used in this lab:
How to Inoculate a Blood Agar Plate and Add a Taxo A (Bacitracin) Disc
How to Interpret Blood Agar with a Taxo A (Bacitracin) Disc; Identification of Streptococcus pyogenes
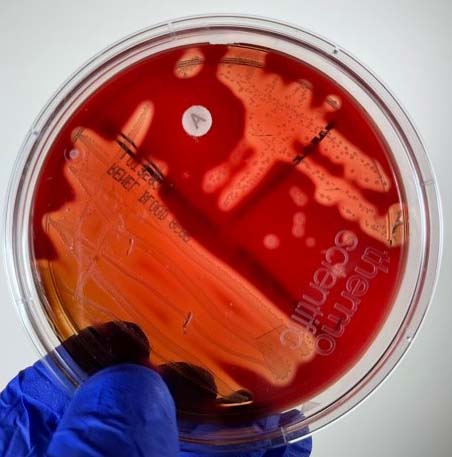
Group A beta streptococci are usually isolated on Blood agar. Streptococcus pyogenes produces
1. Very small, white to grey colonies approximately 1mm in diameter.
2. A zone of beta hemolysis (see Fig. 10) around 2-3mm in diameter surrounding each colony.
There are two streptococcal hemolysins, streptolysin S and streptolysin O. Streptolysin O can be inactivated by oxygen so more distinct hemolysis can be seen by stabbing the agar several times. In this way, some of the organisms form subsurface colonies growing away from oxygen. Since both streptolysin S and streptolysin O are active in the stabbed area, a more clear zone of beta hemolysis can be seen.
3. Sensitivity to the antibiotic bacitracin found in a Taxo A® disk.
Only the group A beta streptococci are sensitive to bacitracin, as shown by a zone of inhibition around a Taxo A® disk ( Fig. 10), a paper disc containing low levels of bacitracin. Any size zone of inhibition will be considered as positive for inhibition to bacitracin. Other serologic groups of streptococci are resistant to bacitracin and show no inhibition around the disk. (The Lancefield group of a group A beta streptococcus can also be determined by direct serologic testing as will be demonstrated in Lab 16.)
Fig. 10: Streptococcus pyogenes Growing on Blood Agar with a Taxo A Disc
Note small, white opaque colonies, beta hemolysis (complete lysis of the red blood cells around the colonies), and sensitivity to the antibiotic bacitracin in the Taxo A® disk. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
See Fig. 9 above for a blood agar plate of a throat culture showing possible Streptococcus pyogenes.
2. The Pneumococcus (Streptococcus pneumoniae)
DISCUSSION
Streptococcus pneumoniae, or the pneumococcus (see Fig. 11), is a lancet-shaped (pointed like a lance) Gram-positive coccus 0.6-1.2 µm in diameter. They typically appear as a diplococcus, but occasionally appear singularly or in short chains. Pneumococci are frequently found as normal flora of the nasopharynx of healthy carriers. Pharyngeal colonization occurs in 40%-50% of healthy children and 20%-30% of healthy adults.
Fig. 11: Encapsulated Streptococcus pneumoniae
Streptococcus pneumoniae, or the pneumococcus, is a Gram-positive lanceolate coccus usually appearing as a diplococcus, but occasionally appearing singularly or in short chains. Pneumococci are frequently found as normal flora of the nasopharynx of healthy carriers. From 10% to 40% of adults carry the bacterium in the nasopharynx. In the U.S., they are the most common cause of community-acquired pneumonia requiring hospitalization, causing around 500,000 cases per year and usually occuring as a secondary infection in the debilitated or immunocompromised host. The pneumococci also cause over 7,000,000 cases of otitis media per year, are the leading cause of sinusitis in people of all ages, are responsible for 500,000 cases of bacteremia, and 3000 cases of meningitis, being the most common cause of meningitis in adults and children over 4 years of age.Note gram-positive encapsulated diplococci. The large cells with the dark red nuclei are while blood cells.
Encapsulated Streptococcus pneumoniae. © Gloria Delisle and Lewis Tomalty, authors. Licensed for use, ASM MicrobeLibrary.
Worldwide, as well as in the U.S., S. pneumoniae remains the most common cause of community-acquired pneumonia, otitis media, bacteremia, and bacterial meningitis. In the U.S., pneumococci are the most common cause of community-acquired pneumonia requiring hospitalization, causing an estimated 500,000 cases per year and usually occuring as a secondary infection in the debilitated or immunocompromised host. The pneumococci also cause between 6 and 7 million cases of otitis media per year, are the leading cause of sinusitis in people of all ages, are responsible for 55,000 cases of bacteremia, and 3000 cases of meningitis, being the most common cause of meningitis in adults and children over 4 years of age.
The capsule serves as the major virulence factor, enabling the pneumococcus to resist phagocytic engulfment, and glycopeptides from its Gram-positive cell wall can lead to excessive cytokine production and a massive inflammatory response.
For further information on virulence factors for Streptococcus pneumoniae, see the following Softchalk lessons:
- Gram-Positive PAMPs
- The Ability to Adhere to Host Cells
- The Ability to Resist Phagocytic Engulfment
- The Ability to Evade Adaptive Immune Defenses
- Type II Toxins that Damage Cell Membranes
- The Ability to Induce Autoimmune Responses
ISOLATION AND IDENTIFICATION OF PNEUMOCOCCI (Streptococcus pneumoniae)
Video lesson - Alpha Hemolysis on Blood Agar and Identification of Streptococcus pneumoniae
1. Isolation on Blood agar
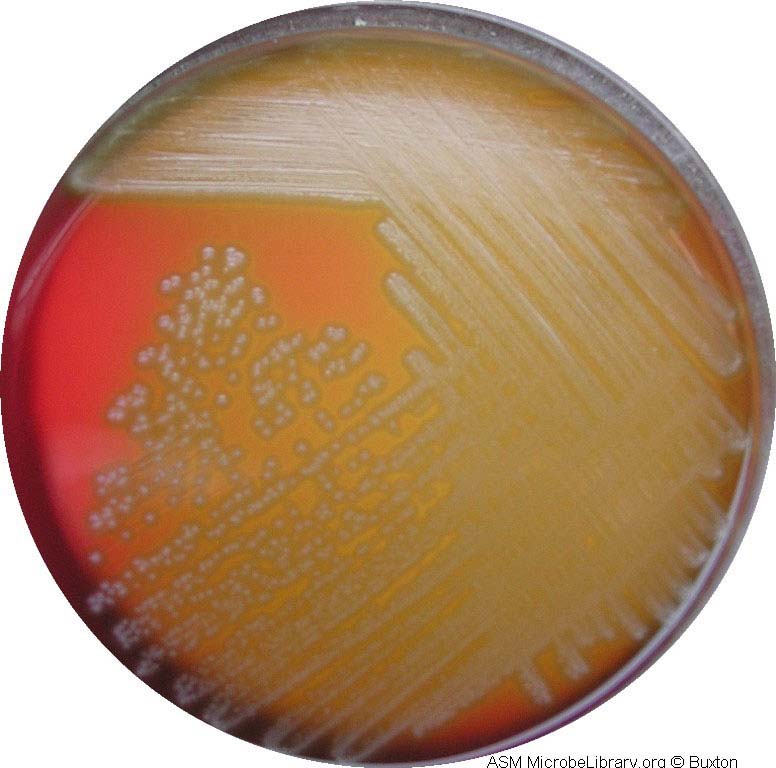
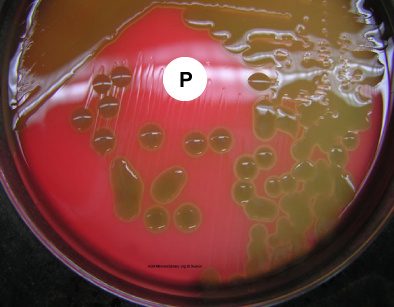
Pneumococci frequently require enriched media and increased CO2 tension for initial isolation. They are usually isolated on Blood agar and incubated in a candle jar (a closed container in which a lit candle is placed to remove O2 and increase CO2 ) at 37C. On Blood agar, colonies appear small, shiny, and translucent. They are surrounded by a zone of alpha hemolysis (see Fig. 12). Due to autolysis with age, the colonies may show a depressed center with an elevated rim.
2. Optochin sensitivity
Pneumococci are the only streptococci that are sensitive to the drug optochin (ethylhydrocupreine hydrochloride). This can be detected by a zone of inhibition around a Taxo P® disk (see Fig. 12), a paper disk containing the drug optochin, which is placed on the Blood agar plate prior to incubation.
Fig. 12: Streptococcus pneumoniae Growing on Blood Agar with a Taxo P Disk (Indirect Lighting)
Note the mucoid colonies, alpha hemolysis (greenish discolorization of the red blood cells around the colonies) and sensitivity to the drug optochin in the Taxo P® disk. Photograph from From MicrobeLibrary.org
Courtesy of Rebecca Buxton, University of Utah
3. Bile solubility test
Most colonies of S. pneumoniae will dissolve within a few minutes when a drop of bile is placed upon them. (This test will not be done in lab today.)
4. Gram stain of sputum
Streptococcus pneumoniae will usually appear as encapsulated, Gram-positive, lancet-shaped diplococci.
3. The Viridans Streptococci
DISCUSSION
Ten species of streptococci are known as the viridans streptococci. They are the dominant normal flora in the upper respiratory tract. Species include S. mutans, S. sanguis, S. mitis, and S. salivarius. S. mutans is the primary cause of dental caries. Viridans streptococci are responsible for between 50% and 70% of the cases of bacterial endocarditis, especially in people with previously damaged heart valves. They are also frequently associated with bacteremia, deep wound infections, dental abscesses, and abscesses of internal organs. The viridans streptococci (see Fig. 13), show alpha hemolysis or no hemolysis on Blood agar, do not possess Lancefield group antigens, and can be differentiated from other alpha streptococci by biochemical testing.
Fig. 13: Alpha Hemolysis on Blood Agar (Indirect Lighting)
Note the partial hemolysis accompanied by a greenish discolorization of the agar around the growth. Photograph from From MicrobeLibrary.org
Courtesy of Rebecca Buxton, University of Utah
DISCUSSION
Enterococci are Gram-positive streptococci that are normal flora of the intestinal tract. They typically occur singly, in pairs, short chains, and clusters, especially when taken off an agar culture for staining. Like the genus Streptococcus, the genus Enterococcus is catalase-negative. Enterococci responsible for a variety of opportunistic infections in humans, and serologically belong to Lancefield group D streptococci.
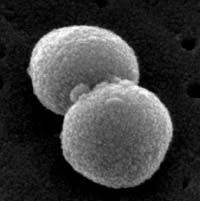
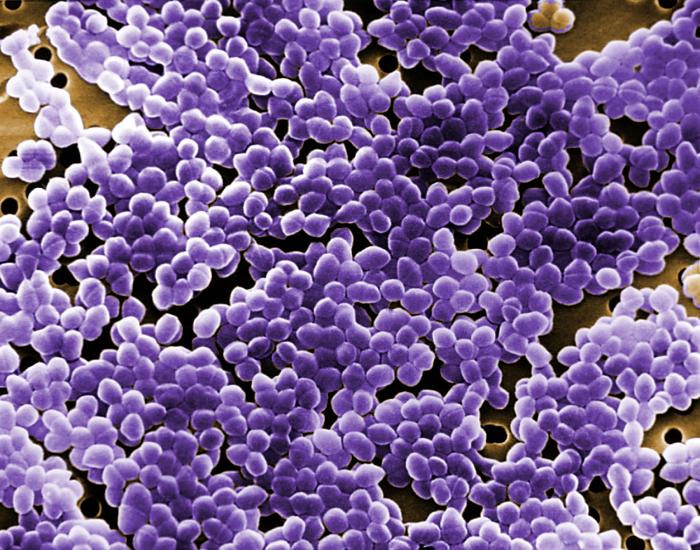
Enterococcus faecalis (see Fig. 14) is the most common enterococcus causing human infections, representing 80-90% of human enterococcal clinical isolates. E. faecalis is normal flora of the intestinal tract in humans and is regularly isolated from infections within the peritoneal cavity (especially following penetrating trauma), urinary tract infections, kidney infections, prostate infections, endocarditis, and infections of damaged or compromised skin such as diabetic or decubitus ulcers, burns and surgical wounds. Trauma to the intestines can lead to intra abdominal and pelvic infections. Other opportunistic enterococcal species include E. faecium and E. durans. The enterococci have become the second most common bacterium isolated from nosocomial urinary and wound infections, and the third most common cause of nosocomial bacteremia. Each year in the U.S., in fact, enterococci account for approximately 110,000 urinary tract infections, 40,000 wound infections, 25,000 cases of nosocomial bacteremia, and 1100 cases of endocarditis. Furthermore, the enterococci are among the most antibiotic resistant of all bacteria, with some isolates resistant to all known antibiotics. The ability of enterococci to produce biofilms protects the organism from the body's defenses as well as promotes exchange of genetic material with other pathogens. A scanning electron micrograph of Enterococcus can be seen in Fig. 15.
Fig. 14: Gram Stain of Enterococcus faecalis in a Blood Culture
Fig. 15: Scanning Electron Micrograph of Enterococcus Species
Enterococcus species are normal glora of the intestinal tract. Enterococcus faecalis frequently causes infections within the peritoneal cavity, especially following penetrating trauma such as gunshot wounds, knife wounds, and surgical wounds, urinary tract infections, kidney infections, prostate infections, and infections of damaged or compromised skin, such as diabetic or decubitus ulcers, burns, and surgical wounds. Other opportunistic fecal streptococci include E. faecium and E. durans. The enterococci have become the second most common bacterium isolated from nosocomial urinary and wound infections, and the third most common cause of nosocomial bacteremia.Furthermore, the enterococci are among the most antibiotic resistant of all bacteria, with some isolates resistant to all known antibiotics. Note Gram-positive streptococci. Note streptoococcus arrangement (cocci in chains). Image: Enterococcus faecalis in a Blood Culture. © Gloria Delisle and Lewis Tomalty, authors.
Licensed for use, ASM MicrobeLibrary.By Content Providers(s): CDC/ Janice Haney Carr [Public domain] Courtesy of the Centers for Disease Control and Prevention.
ISOLATION AND IDENTIFICATION OF ENTEROCOCCI
The enterococci may be isolated and identified using various selective and differential media.
Bile Esculin Azide Agar
Unlike most bacteria, the enterococci will grow in the presence of the bile salts in the medium. The sodium azide in the medium inhibits the growth of Gram-negative bacteria. Enterococci will hydrolyze the esculin, producing esculetin which reacts with the iron salts in the medium turning the agar black (see Fig. 16A and 16B).
Fig. 16A: A Plate of Uninoculated Bile Esculin Azide Agar
Fig. 16B: Enterococcus faecalis Growing on Bile Esculin Azide Agar
Enterococcus faecalis growing on a Bile Esculin Azide Agar plate showing the hydrolysis of esculin which turns the agar black. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
On blood agar, most strains of Enterococcus faecalis show gamma reaction on sheep blood agar, however some strains exhibit beta hemolysis. Colonies are usually 1-2 millimeters in diameter. Enterococci are also being identified using chemiluminescent labelled DNA probes complementary to species-specific bacterial ribosomal RNA (rRNA) sequences.
Case Study #1
Choose either unknown #1 or unknown #2 as your unknown for Case Study #1.
A 21-year old male complains of a sore throat and painful swallowing. A physical exam of the throat shows tonsillopharyngeal edema and erythema, a patchy exudate, petechiae on the soft palate, and a red, swollen uvula. He has a temperature of 101.6 °F. He doesn't have a cough or a noticeably runny nose.
Assume that your unknown is a transport medium from a swab of this person's throat.
CAUTION: TREAT EACH UNKNOWN AS A PATHOGEN!. Inform your instructor of any spills or accidents. WASH AND SANITIZE YOUR HANDS WELL before leaving the lab.
MATERIALS
1 plate of blood agar, 1 Taxo A ® disc, 1 sterile swab, inoculating loop
PROCEDURE (to be done in groups of 3)
Videos reviewing techniques used in this lab:
How to Inoculate a Blood Agar Plate and Add a Taxo A (Bacitracin) Disc
How to Interpret Blood Agar with a Taxo A (Bacitracin) Disc; Identification of Streptococcus pyogenes
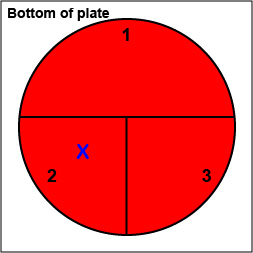
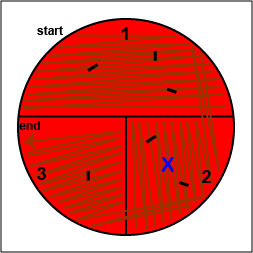
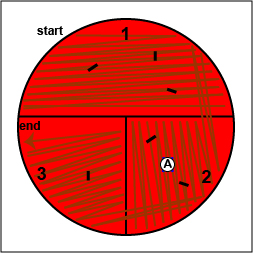
1. On the bottom of the petri plate, divide the plate into thirds with your wax marker and label as shown below. Before you streak your plate draw an "X" on the bottom of the blood agar plate in sector 2 to indicate where you will eventually place the Taxo A disk as indicated in Fig. 17, step 1.
Fig. 17: Inoculating a Blood Agar Plate with your Unknown, Step 1 Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
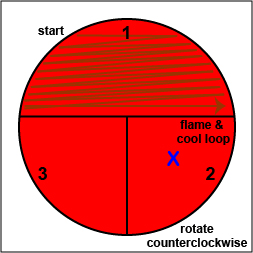
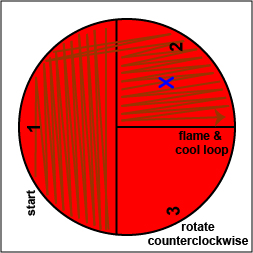
2. Using a sterile inoculating loop, streak your unknown for isolation on a blood agar plate so as to get single, isolated colonies (see Fig. 17, step 2, Fig. 17, step 3, and Fig. 17, step 4).
Fig. 17: Inoculating a Blood Agar Plate with your Unknown, Step 2 Fig. 17: Inoculating a Blood Agar Plate with your Unknown, Step 3 Fig. 17: Inoculating a Blood Agar Plate with your Unknown, Step 4 Using a sterile inoculating loop, streak one-third of the blood agar plate with your unknown. Flame the loop and let it cool. Rotate the plate counterclockwise so sector 1 is at 9:00. Using a sterile inoculating loop, spread out some of the bacteria in area 1 over area 2. Flame the loop and let it cool. Rotate the plate counterclockwise so sector 1 is at 9:00. Using a sterile inoculating loop, spread out some of the bacteria in area 2 over area 3. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
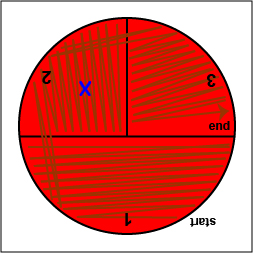
3. Using your inoculating loop, stab the agar 2-3 times in each of the growth areas in order to detect oxygen-sensitive hemolysins (see Fig. 17, step 5).
Fig. 17: Inoculating a Blood Agar Plate with your Unknown, Step 5 Stab the agar 2-3 times in each if the growth areas. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
4. Place a Taxo A ® disk containing bacitracin where you drew the "X" in sector 2. (see Fig. 17, step 6). Tap it lightly with your loop so that the disk sticks to the agar.
Fig. 17: Inoculating a Blood Agar Plate with your Unknown, Step 6 Place a Taxo A disk over the "X" in sector 2 and tap it lightly with your loop so that it sticks to the agar. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
5. Incubate the blood agar plate upside down and stacked in the petri plate holder on the shelf of the 37°C incubator corresponding to your lab section until the next lab period.
Case Study #2
Choose unknown #3 as your unknown for Case Study #2.A 57-year old diabetic male hospitalized following hip replacement surgery has had an indwelling urinary catheter inserted for 8 days. He presents with suprapubic discomfort. His blood pressure is normal and he does not have fever, chills, or flank pain. There is no costovertebral angle (CVA) tenderness. A complete blood count (CBC) shows leukocytosis with a left shift. A urine dipstick shows a positive leukocyte esterase test, a negative nitrite test, 30mg of protein per deciliter, and red blood cells in the urine. Microscopic examination of centrifuged urine shows 50 white blood cells, as well as 20 bacteria and 5 red blood cells per high-power field.
Assume that your unknown is from the urine of this patient.
CAUTION: TREAT EACH UNKNOWN AS A PATHOGEN!. Inform your instructor of any spills or accidents. WASH AND SANITIZE YOUR HANDS WELL before leaving the lab.
MATERIALS
1 bile esculin agar plate, materials to perform a Gram stain, inoculating loop
PROCEDURE (to be done in groups of 3)
[Keep in mind that in a real clinical situation other lab tests and cultures for bacteria other than those upon which this lab is based would also be done.]
| How to Chemically Fix a Microscope Slide with Methanol |
| How to Prepare a Slide for Staining when using Bacteria from an Agar Culture |
| How to Make a Gram Stain |
| A Review of the Critical Decolorization Step of the Gram Stain |
1. Do a Gram stain on the unknown (see Lab 6). Make sure you review the instructions before you do the Gram stain. Because Enterococci and Staphylococci can sometimes look similar in Gram stains done from a plate culture, perform a catalase test on your unknown to help differentiate an Enterococcus from a Staphylococcus (see Lab 8).