Fungi are eukaryotic organisms and include the yeasts, molds, and fleshy fungi. Yeasts are microscopic, unicellular fungi; molds are multinucleated, filamentous fungi (such as mildews, rusts, and common household molds); the fleshy fungi include mushrooms and puffballs.
All fungi are chemoheterotrophs, requiring organic compounds for both an energy and carbon source, which obtain nutrients by absorbing them from their environment. Most live off of decaying organic material and are termed saprophytes. Some are parasitic, getting their nutrients from living plants or animals.
The study of fungi is termed mycology and the diseases caused by fungi are called mycotic infections or mycoses.
In general, fungi are beneficial to humans. They are involved in the decay of dead plants and animals (resulting in the recycling of nutrients in nature), the manufacturing of various industrial and food products, the production of many common antibiotics, and may be eaten themselves for food. Some fungi, however, damage wood and fabrics, spoil foods, and cause a variety of plant and animal diseases, including human infections.
YEASTS
DISCUSSION
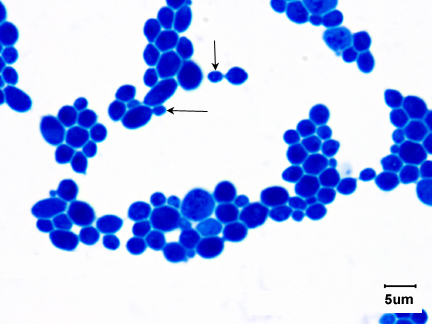
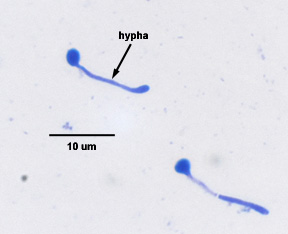
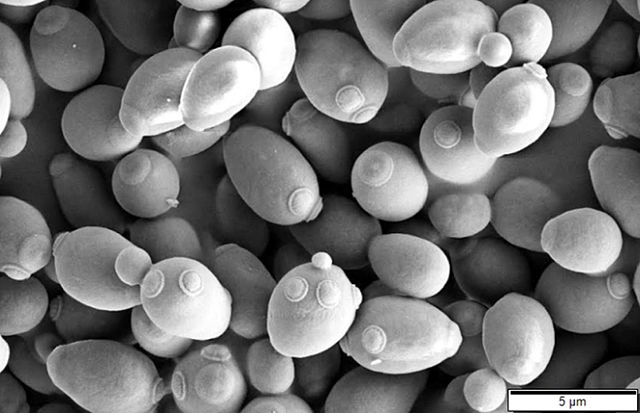
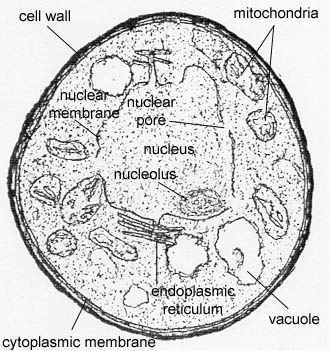
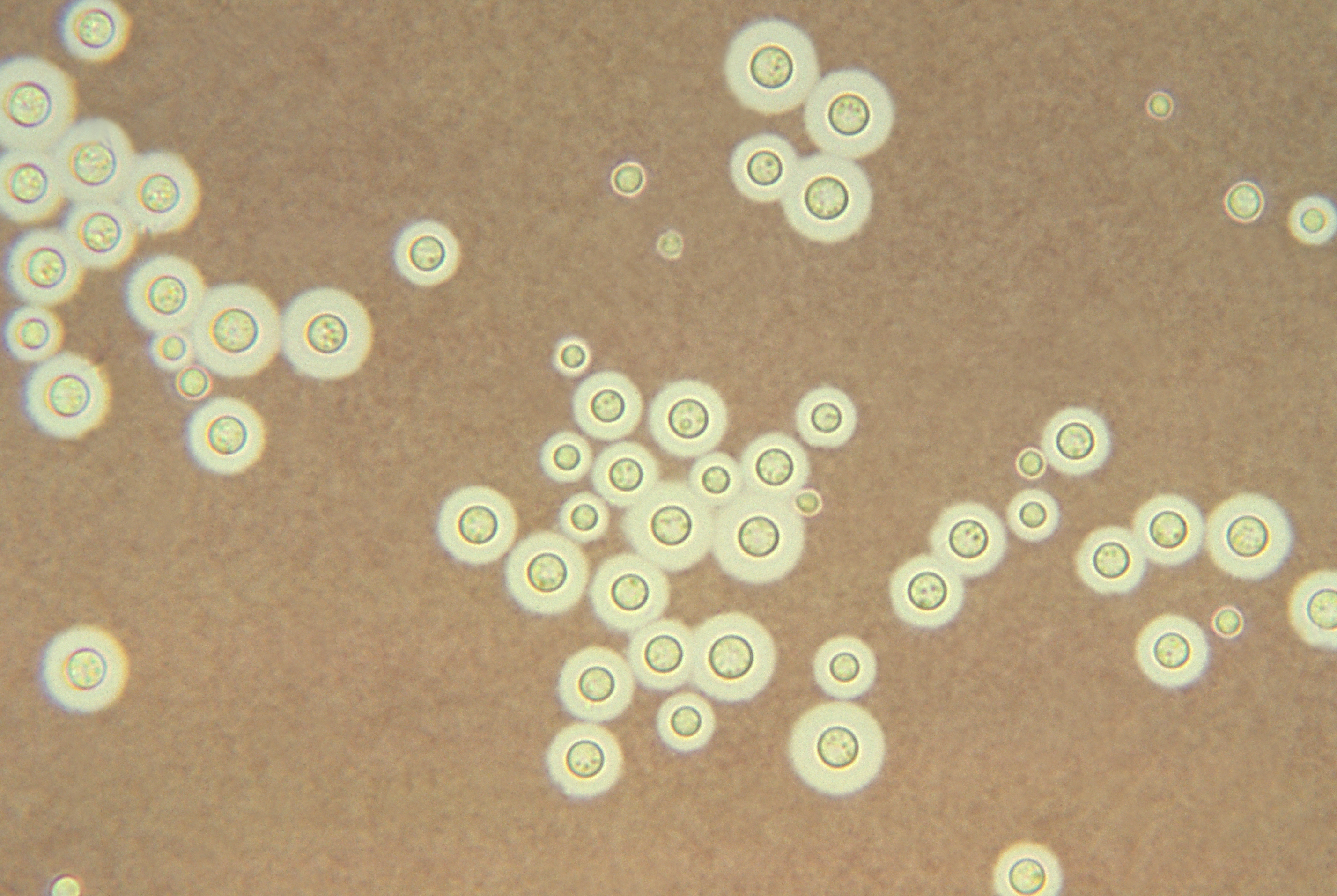
Yeasts are unicellular, oval or spherical fungi which increase in number asexually by a process termed budding (See Fig. 1 and Fig. 3). A bud forms on the outer surface of a parent cell, the nucleus divides with one nucleus entering the forming bud, and cell wall material is laid down between the parent cell and the bud. Usually the bud breaks away to become a new daughter cell but sometimes, as in the case of the yeast Candida, the buds remain attached forming fragile branching filaments called hyphae (See Fig. 2). Because of their unicellular and microscopic nature, yeast colonies appear like bacterial colonies on solid media. It should be noted that certain dimorphic fungi (see Lab 10) are able to grow as a yeast or as a mold, depending on growth conditions. Yeast, as mentioned above, are eukaryotic as seen in Fig. 4.
Fig. 1: Saccharomyces cerevisiae
Fig. 2: Candida albicans Producing a Hypha
Fig. 3: Scanning Electron Micrograph of Saccharomyces cerevisiae Fig. 4: Transmission Electron Micrograph of Candida albicans
Note budding yeast (arrows)
Dimorphic Candida albicans switching from a yeast form to a filamentous hyphal form.
Note forming buds and bud scars. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
By Mogana Das Murtey and Patchamuthu Ramasamy - [1], CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=52254246 Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Developmental Biology Film Series episode 57. The reproduction of yeast cells by budding.
Yeasts are facultative anaerobes and can therefore obtain energy by both aerobic respiration and anaerobic fermentation. The most yeasts are non-pathogenic and some are of great value in industrial fermentations. For example, Saccharomyces species are used for both baking and brewing.
A. Candida
The yeast Candida is normal flora of the gastrointestinal tract and is also frequently found on the skin and on the mucous membranes of the mouth and vagina. Candida is normally held in check in the body by:
1. The body's normal immune defenses; and
2. The body's normal bacterial microbiota.
However, Candida may become an opportunistic pathogen and overgrow an area of colonization if the host becomes immunosuppressed or is given broad-spectrum antibiotics that destroy the normal bacterial microbiota. (Since Candida is eukaryotic, antibiotics used against prokaryotic bacteria do not affect it.)
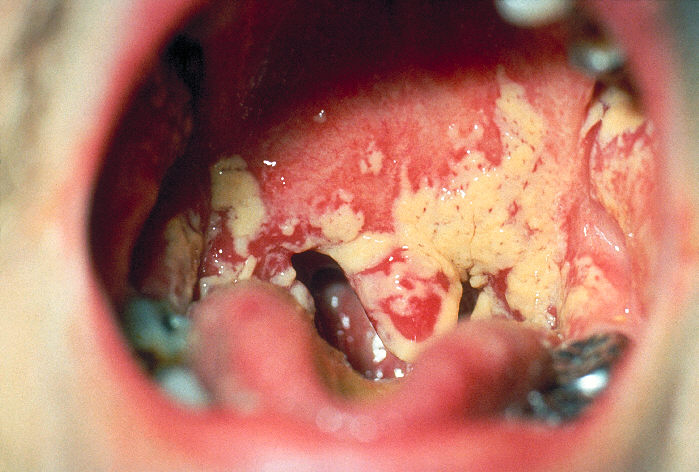
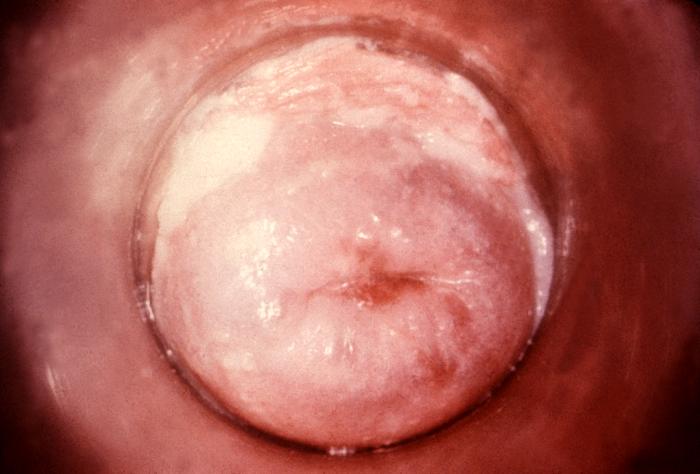
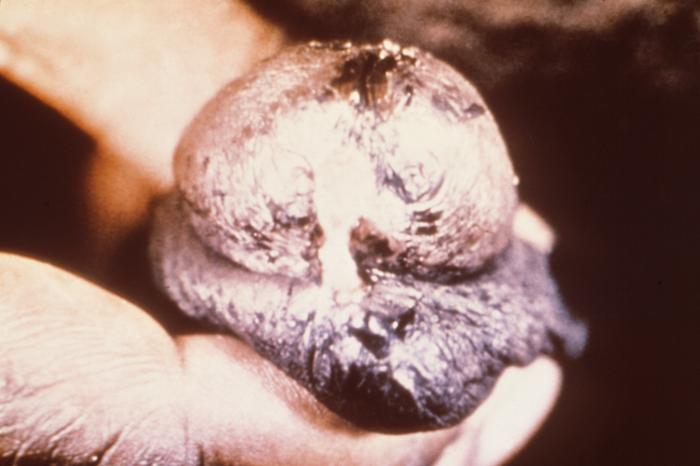
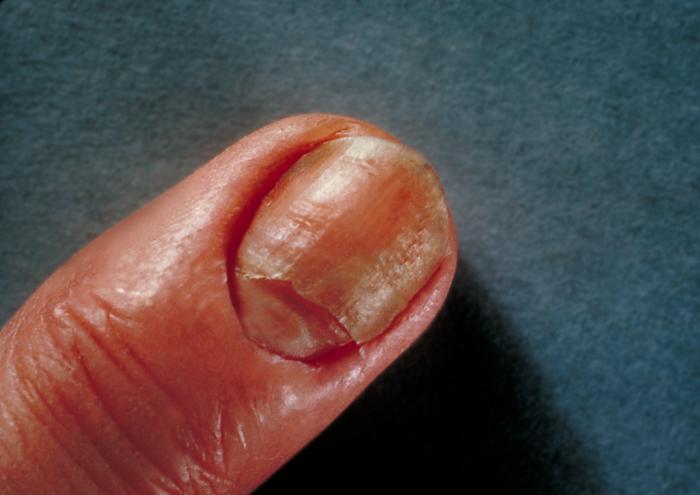
Any infection caused by the yeast Candida is termed candidiasis. The most common forms of candidiases are oral mucocutaneous candidiasis, or thrush (see Fig. 5A and Fig. 5B), vaginitis (see Fig. 5C and 5D), balantitis (infection of the penis, Fig. 5E), onychomycosis (infection of the nails; Fig. 5F), and dermatitis (diaper rash and other infections of moist skin). In addition, Candida can cause urinary tract infections. However, antibiotic therapy, cytotoxic and immunosuppressive drugs, and immunosuppressive diseases such as diabetes, leukemias, and AIDS can enable Candida to cause severe opportunistic systemic infections involving the skin, lungs, heart, and other organs. In fact, Candida now accounts for 10% of the cases of septicemia. Candidiasis of the esophagus, trachea, bronchi, or lungs, in conjunction with a positive HIV antibody test, is one of the indicator diseases for AIDS.
Fig. 5A:Candida albicans growing in the Mouth (Thrush) Fig. 5B: Mouth Smear of a Person with Thrush
Fig. 5C: Candida albicans growing on the Cervix
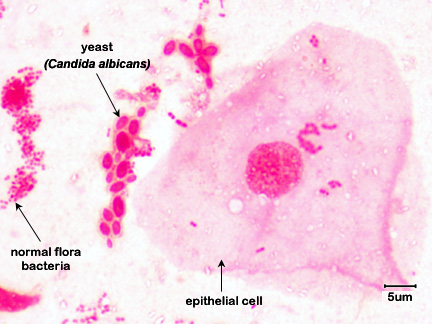
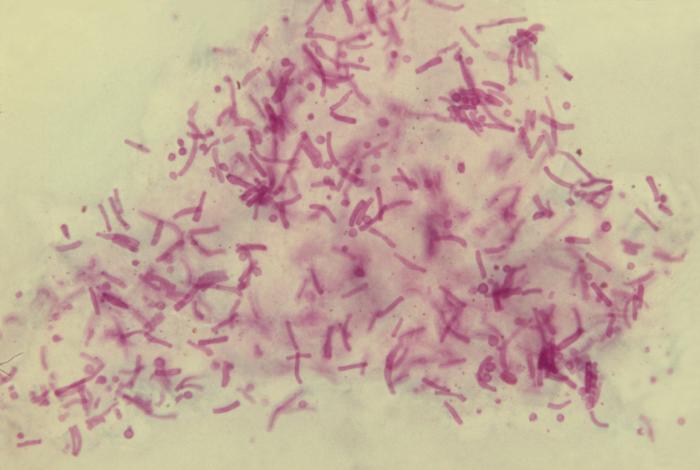
Fig. 5D: Vaginal Smear of a Person with Candida Vaginitis
Note budding yeast (arrows).>
Note epithelial cells, rod-shaped bacteria, and Candida albicans in its hyphal form.By Content Providers: CDC [Public domain].
Courtesy of the Centers for Disease Control and Prevention.Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
By Content Providers: CDC [Public domain].
Courtesy of the Centers for Disease Control and Prevention.Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Fig. 5E:Candida albicans Infection of the Penis (Balantitis)
Fig. 5F: Candida albicans Infection of the Nails By Content Providers: CDC/Richard O. Detrick [Public domain].
Courtesy of the Centers for Disease Control and Prevention.By Content Providers: CDC/Sherry Brinkman [Public domain].
Courtesy of the Centers for Disease Control and Prevention.
The most common Candida species causing human infections is C. albicans, causing 50-60% of all Candida infections. Candida glabrata is second, causing 15-20% of Candida infections; Candida parapsilosis is third, responsible for 10-20%. More recently, Candida auris has been causing relatively rare but severe infections in patients that have been hospitalized for long periods of time, sometimes entering the bloodstream causing invasive infections throughout the body. C. auris is often resistant to common antifungal drugs and kills as many as 1 out of 3 patients with invasive infections.
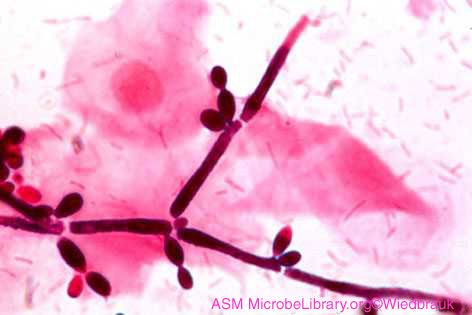
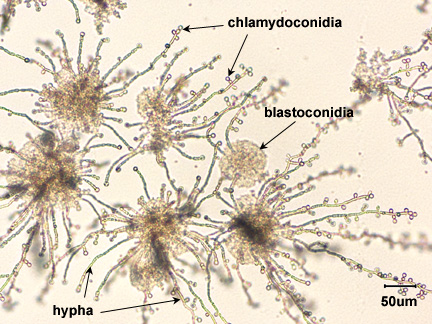
Candida is said to be dimorphic, that is it has two different growth forms. It can grow as an oval, budding yeast, but under certain culture conditions, the budding yeast may elongate and remain attached producing filament-like structures called pseudohyphae. C. albicans may also produce true hyphae similar to molds. In this case long, branching filaments lacking complete septa form. The pseudohyphae and hyphae help the yeast to invade deeper tissues after it colonizes the epithelium. Asexual spores called blastoconidia (blastospores) develop in clusters along the hyphae, often at the points of branching. Under certain growth conditions, thick-walled survival spores called chlamydoconidia (chlamydospores) may also form at the tips or as a part of the hyphae. (See Fig. 6.)
Fig. 6: Candida albicans in its Hyphal Form
Note hyphae, blastoconidia, and chlamydoconidia. Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
B. Cryptococcus neoformans
A lesser known but often more serious pathogenic yeast is Cryptococcus neoformans. Like many fungi, this yeast can also reproduce sexually and the name given to the sexual form of the yeast is Filobasidiella neoformans. It appears as an oval yeast 5-6 µm in diameter, forms buds with a thin neck, and is surrounded by a thick capsule. It does not produce pseudohyphae and chlamydospores. The capsule enables the yeast to resist phagocytic engulfment. The yeast is dimorphic. In its sexual form, as well as in its asexual form under certain conditions, it can produce a hyphal form.
Cryptococcus infections are usually mild or sub-clinical but, when symptomatic, usually begin in the lungs after inhalation of the yeast in dried bird feces. It is typically associated with pigeon and chicken droppings and soil contaminated with these droppings. Cryptococcus, found in soil, actively grows in the bird feces but does not grow in the bird itself. Usually the infection does not proceed beyond this pulmonary stage. However, in an immunosuppressed host it may spread through the blood to the meninges and other body areas, often causing cryptococcal meningoencephalitis. Any disease by this yeast is usually called cryptococcosis.
Dissemination of the pulmonary infection can result in severe and often fatal cryptococcal meningoencephalitis. Cutaneous and visceral infections are also found. Although exposure to the organism is probably common, large outbreaks are rare, indicating that an immunosuppressed host is usually required for the development of severe disease. Extrapulmonary cryptococcosis, in conjunction with a positive HIV antibody test, is another indicator disease for AIDS. People with AIDS-associated cryptococcal infections account for 80%-90% of all patients with cryptococcosis.
Cryptococcus can be identified by preparing an India ink or nigrosin negative stain of suspected sputum or cerebral spinal fluid in which the encapsulated, budding, oval yeast cells (see Fig. 7) may be seen. It can be isolated on Saboraud Dextrose agar and identified by biochemical testing. Direct and indirect serological tests (discussed in Labs 16) may also be used in diagnosis.
Fig. 7: India ink stain of encapsulated Cryptococcus neoformans
Note encapsulated yeast. By Content Providers: CDC/Dr. Leanor Haley [Public domain].
Courtesy of the Centers for Disease Control and Prevention.
C. Pneumocystis jiroveci
Pneumocystis jiroveci, (formerly called Pneumocystis carinii ), causes Pneumocystis pneumonia (PCP). It is seen almost exclusively in highly immunosuppressed individuals such as those with AIDS, late -stage malignancies, or leukemias. PCP is one of the more common infections associated with AIDS.
P. jiroveci can be found in 3 distinct morphologic stages:
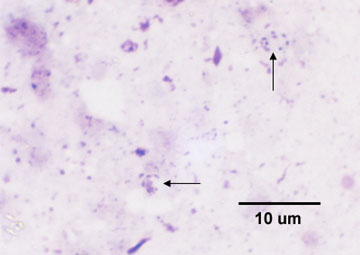
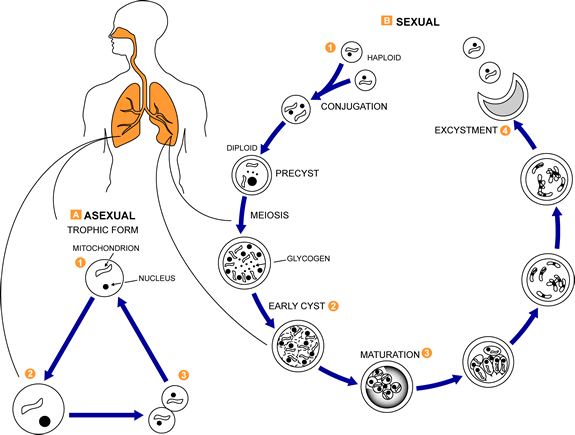
- The trophozoite (trophic form), a haploid amoeboid form 1-4 µm in diameter that replicates by mitosis and binary fission. The trophic forms are irregular shaped and often appears in clusters. See Fig. 8A.
- A precystic form or early cyst. Haploid trophic forms conjugate and produce a diploid precyst form or sporocyte.
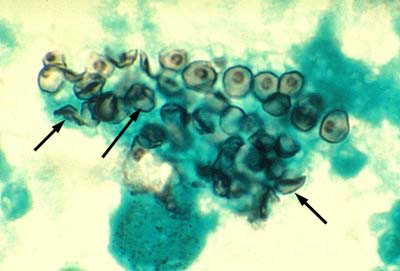
- The precyst form matures into a cyst form, which contains several intracystic bodies or spores are 5-8 µm in diameter. It has been postulated that in formation of the cyst form (late phase cyst), the zygote undergoes meiosis and subsequent mitosis to typically produce eight haploid ascospores (sporozoites) See Fig. 8B. As the haploid ascospores are released the cysts often collapse forming crescent-shaped bodies (see Fig. 8C). P. jiroveci is usually transmitted by inhalation of the cyst form. Released ascospores then develop into replicating trophic forms that attach to the wall of the alveoli and replicate to fill the alveoli.
- A proposed life cycle for Pneumocystis jiroveci can be seen in Fig. 8D.
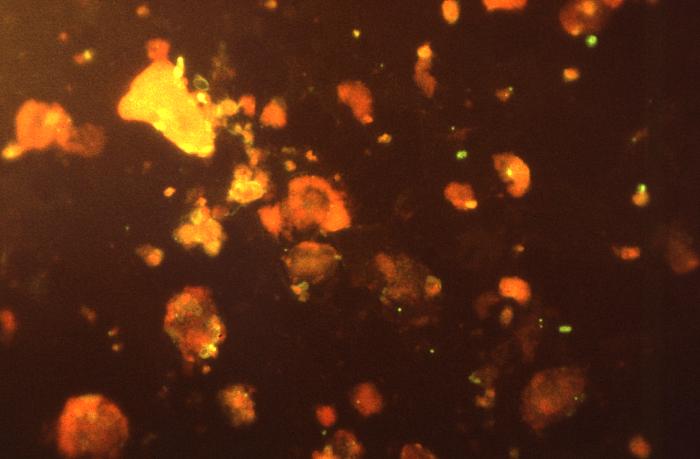
Fig. 8A: Trophic Form of Pneumocystis jiroveci from the Lungs of a Person with PCP
Fig. 8B: Cysts of Pneumocystis jiroveci from the Lungs of a Person with PCP
Fig. 8C: Cysts of Pneumocystis jiroveci in Smear from Bronchoalveolar Lavage
Cysts of Pneumocystis jiroveci from the Lungs of a Person with PCP
Cysts of Pneumocystis jiroveci in bronchoalveolar material, Giemsa stain method.The rounded cysts (arrows) are 4 to 7 µm in diameter and contain 6 to 8 intracystic bodies, whose nuclei are stained by the dye. The walls of the cysts are not stained.
Cysts of Pneumocystis jiroveci in lung tissue, Gomori methenamine silver stain method. The walls of the cysts are stained black and often appear crescent shaped or like crushed ping-pong balls. The intracystic bodies are not visible with this stain.By Content Providers: CDC/Lois Norman [Public domain].
Courtesy of the Centers for Disease Control and Prevention.Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
By Content Providers: CDC [Public domain].
Courtesy of the Centers for Disease Control and Prevention.
Fig. 8D: Proposed life cycle for Pneumocystis jiroveci
By Content Providers: CDC [Public domain].
Courtesy of the Centers for Disease Control and Prevention.
In biopsies from lung tissue or in tracheobronchial aspirates, both a trophic form about 1-4 µm in diameter with a distinct nucleus and a cyst form between 5-8 µm in diameter with 6-8 intracystic bodies (ascospores) can be seen.
When viewing cysts of P. jiroveci in lung tissue after utilizing the Gomori methenamine silver stain method, the walls of the cysts are stained black and often appear crescent shaped or like crushed ping-pong balls, as seen in Fig. 8C above.The intracystic bodies are not visible with this stain.
D. Malassezia globosa
Malassezia globosa is a dimorphic yeast (see Fig. 9)that is the most frequent cause of a superficial skin infection called tinea versicolor that commonly appears as a hypopigmentation of the infected skin. M. globosa is also the most common cause of dandruff and seborrheic dermatitis. The yeast is naturally found on the skin.
Fig. 9: Malassezia Species in its Dimorphic Form
Note both the yeast form and the hyphal form. By Content Providers: CDC/Dr. Lucille K. Georg [Public domain].
Courtesy of the Centers for Disease Control and Prevention.
For a description of antifungal agents used to treat fungal infections, see Chemotherapeutic Control of Fungi in my CourseArc Lecture Lessions.
Medscape articles on infections associated with organisms mentioned in this lab exercise. Registration to access this website is free.
Today we will use three agars to grow our yeast: Saboraud Dextrose agar (SDA), Mycosel agar, and Rice Extract agar. Saboraud Dextrose agar (SDA) is an agar like trypticase soy agar but with a higher sugar concentration and a lower pH, both of which inhibit bacterial growth but promote fungal growth. SDA, therefore, is said to be selective for fungi.
Another medium, Mycosel agar, contains chloramphenicol to inhibit bacteria and cycloheximide to inhibit most saprophytic fungi. Mycosel agar, therefore, is said to be selective for pathogenic fungi.
Rice Extract agar with polysorbate 80 stimulates the formation of hyphae, blastoconidia, and chlamydoconidia (see Fig. 11), structures unique to C. albicans, and may be used in its identification. The speciation of Candida is based on sugar fermentation patterns.
Fig. 11: Candida albicans in its Hyphal Form
Note hyphae, blastoconidia (blastospores), and chlamydoconidia (chlamydospores).Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
MATERIALS
Coverslips, alcohol, forceps, and one plate each of Saboraud Dextrose agar, Mycosel agar, and Rice Extract agar.
ORGANISMS
Trypticase Soy broth cultures of Candida albicans and Saccharomyces cerevisiae.
PROCEDURE (to be done in pairs)
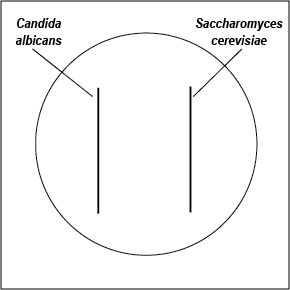
1. With a wax marker, divide a Saboraud Dextrose agar and a Mycosel agar plate in half. Using a sterile swab, inoculate one half of each plate with C. albicans and the other half with S. cerevisiae as shown in Fig. 12. Incubate the two plates upside down and stacked in the petri plate holder on the shelf of the 37°C incubator corresponding to your lab section until the next lab period.
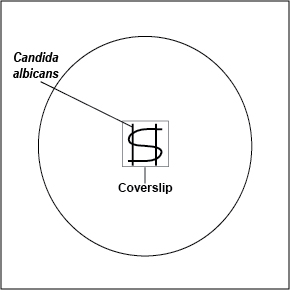
2. Using your inoculating loop, streak two parallel lines of Candida albicans approximately 1.5 cm long and 1.0 cm apart onto the surface of a plate of Rice Extract agar. Sterilize the inoculating loop and let it cool. Using the sterile loop, make an S-shaped streak lightly back and forth across the two parallel streak lines as shown in Fig. 13. Pick up a glass coverslip with forceps, dip the coverslip in alcohol, and ignite with the flame of your butane lighter. Let the coverslip cool for a few seconds and place it over a portion of the streak line so that the plate can be observed directly under the microscope after incubation. Incubate upside down at room temperature for 3-5 days and examine microscopically.
Fig. 12: Inoculating SDA and Mycosel Agar Plates with Candida albicans and Saccharomyces cerevisiae
Fig. 13: Inoculating a Rice Extract Agar Plate with Candida albicans
Streak with sterile swabs and incubate upside down and stacked in the petri plate holder on the shelf of the 37°C incubator corresponding to your lab section.
Using your inoculating loop, streak two parallel lines approximately 1.5 cm long and 1.0 cm apart onto the surface of a plate of Rice Extract agar. Make an S-shaped streak lightly back and forth across the two parallel streak lines. Pick up a glass coverslip with forceps, dip the coverslip in alcohol, and ignite with the flame of your butane lighter. Let the coverslip cool for a few seconds and place it over a portion of the streak line so that the plate can be observed directly under the microscope after incubation. Incubate upside down at room temperature.Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
Gary E. Kaiser, Ph.D.
Professor of Microbiology
The Community College of Baltimore County, Catonsville Campus
This work is licensed under a Creative Commons Attribution 3.0 Unported License
3. Observe the following demonstrations:
a. Direct stain of Saccharomyces cerevisiae
b. Direct stain of Candida albicans
c. Oral smear from a person with thrush.
d. Lung tissue infected with Candida albicans
RESULTS
1. In the table below, describe the appearance of Candida albicans and Saccharomyces cerevisiae on Saboraud Dextrose agar.
Also in the table below, describe the appearance of Candida albicans and Saccharomyces cerevisiae on Mycosel agar.
Yeast SDA Mycosel agar Candida albicans Saccharomyces cerevisiae
2. Remove the lid of the Rice Extract agar plate and put the plate on the stage of the microscope. Using your yellow-striped 10X objective, observe an area under the coverslip that appears "fuzzy" to the naked eye. Reduce the light by moving the iris diaphragm lever almost all the way to the right. Raise the stage all the way up using the coarse focus (large knob) and then lower the stage using the coarse focus until the yeast comes into focus. Draw the hyphae, blastoconidia, and chlamydoconidia. See lab 1 for focusing instructions using the 10X objective.
3. Observe and make drawings of the demonstration yeast slides.
PERFORMANCE OBJECTIVES FOR LAB 9
After completing this lab, the student will be able to perform the following objectives:
INTRODUCTION
1. Define mycology and mycosis.
2. State three ways fungi may be beneficial to humans and three ways they may be harmful.
DISCUSSION
1. Describe the typical appearance of a yeast cell and its usual mode of reproduction.
2. Describe yeasts in terms of their oxygen requirements.
3. State two ways the yeast Saccharomyces is beneficial to humans.
4. Name three yeasts that commonly infect humans.
5. Name four common forms of candidiasis.
6. Describe two conditions that may enable Candida to cause severe opportunistic systemic infections.
7. Describe pseudohyphae, hyphae, blastoconidia (blastospores), and chlamydoconidia (chlamydospores).
8. State the usefulness of Saboraud Dextrose agar, Mycosel agar, and Rice Extract agar.
9. State how Cryptococcus neoformans is transmitted to humans, where in the body it normally infects, and possible complications.
10. State the primary method of identifying Cryptococcus neoformans when causing cryptococcal meningoencephalitis.
11. State what disease is caused by Pneumocystis jiroveci and indicate several predisposing conditions a person is normally seen to have before they contract the disease.
12. Name an infection caused by Malassezia globosa.
RESULTS
1. Describe the appearance of Saccharomyces cerevisiae and Candida albicans on Saboraud Dextrose agar and on Mycosel agar.
2. When given a plate of Mycosel agar showing yeast-like growth and a plate of Rice Extract agar showing hyphae, blastosconidia (blastospores), and chlamydoconidia (chlamydospores), identify the organism as Candida albicans.
3. Recognize the following observed microscopically:
a. Saccharomyces cerevisiae and Candida albicans as yeasts in a direct stain preparation
b. A positive specimen for thrush by the presence of budding Candida albicans
c. Cryptococcus neoformans in an India ink preparation
d. Pneumocystis jiroveci in lung tissue
SELF-QUIZ
Microbiology Laboratory Manual by Gary E. Kaiser, PhD, Professor of Microbiology
is licensed under a Creative Commons Attribution 4.0 International License.
Last updated: April, 2023